If not identified as being innocuous, they can lead to entire raw material batches being rejected — unnecessarily and often at high costs — by users. Still, there are no official guidelines available regarding how to deal with these foreign particles. Proactively managing and communicating TUPs with the help of dedicated TUP profiles could be the answer.
The presence of foreign matter in excipients and other chemical raw materials is typically considered to be a threat to the final pharmaceutical product’s quality. In fact, particles can endanger patient safety. They can also violate compendia and regulatory requirements, disrupt supply chains, lead to drug shortages and harm a company’s reputation.
However, not all particles are dangerous. Technically unavoidable particles (TUPs), as defined by International Pharmaceutical Excipient Council (IPEC), which can arise during excipient manufacturing, handling or packaging, neither pose a higher risk to patient safety, nor do they affect the efficacy or quality of the drug product.1
Unavoidable remains unavoidable
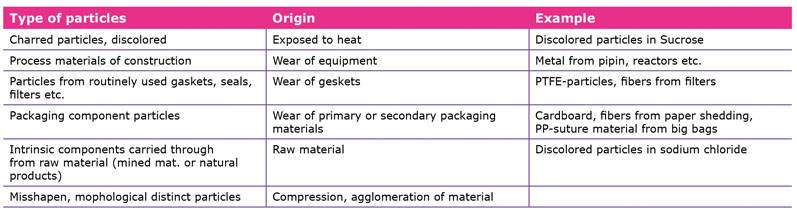
TUPs have always been present in excipients (Table I). In recent years, however, the concern regarding their presence has increased dramatically. IPEC assumes that the increased concern stems from several US FDA warning letters to pharmaceutical companies, which highlight adverse findings during inspection and cite the insufficient or incomplete investigation of unusual, visible particles.
As the FDA comments: “There are risk-based, science-based decisions that you need to make as the manufacturer of the final drug about what you can tolerate in your incoming raw materials.”2
The FDA goes on to say: “Perhaps it is as simple as having a discussion with your supplier and being able to explain to the agency what the particles are and why they are not a risk to public or patient safety.”
This, however, is easier said than done. Instead, the warning letters have created a great deal of uncertainty in the industry and have led to the unrealistic expectation that not a single visible particle should be present in any amount of active pharmaceutical ingredient (API), irrespective of its size or the overall amount present in a batch.
Yet TUPs are, as their name indicates, unavoidable. They cannot be eliminated in an economically viable manner using current technologies. Instead, managing them proactively and informing customers about their presence and their respective characteristics helps to differentiate between issues that warrant action … and those that do not.
Proactive communication is the key
Specific guidance from regulatory authorities regarding how to handle the presence of TUPs in APIs would help all parties involved … and some attempts have already been made. The guidelines developed by the Active Pharmaceuticals Ingredients Committee (APIC) provide a common understanding concerning the presence of particles.
They share possible tools to help systematically identify the root causes, define appropriate corrective or preventive actions and conduct risk assessments. They also describe proven testing methods, scientifically based acceptance criteria and provide the latest scientific, process, analytical, equipment and engineering-related findings, as well as good practices to minimise the presence and risk of foreign particles in APIs.
IPEC released another set of guidelines after the previously mentioned FDA warning letters. They describe a way to supply data on the identity and origin of particles in excipients. Most importantly, they encourage communication between excipient manufacturers and users to reduce the amount of time, money and resources involved, and to ensure adequate investigation.
Hence, the FDA has been proven right in one very important regard: open communication is crucial when handling TUPs. Experience shows that proactively searching for these foreign particles and providing customers with information supports risk mitigation. TUP profiles are a tried and trusted means of open communication and have proved to be especially helpful for both excipient and pharmaceutical manufacturers.
Creating TUP profiles
Based on the experience of suppliers and users of chemical raw materials and excipients, Merck KGaA, based in Darmstadt, Germany, has implemented processes to proactively provide customers with information regarding TUPs for their Emprove excipients.
The goal is to inform them about the presence of TUPs and to provide details on the corrective and preventive action (CAPA) taken and the mitigation strategies that are used. The root cause analysis on the particles’ source saves customers time and resources and enables them to independently assess the potential impact of particulate matter in their products (see Table II).
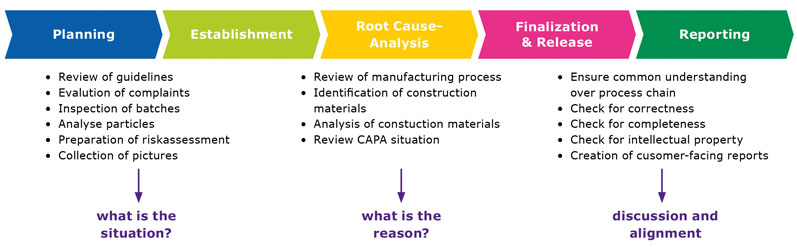
The first step in preparing a TUP profile consists of analysing the situation. This includes inspecting batches and evaluating complaints. Which products have received more complaints about foreign particles than others? Once the TUPs have been detected, assessed and inspected, the corresponding guidelines (if available) are reviewed.
When it comes to metal impurities, for instance, the ICH Q3D guidelines for elemental impurities are a good starting point. Step two consists of a root cause analysis to identify the origin of the particles. Can they be avoided entirely or at least reduced?
To answer this question, the production process is reviewed and construction material is identified. Apart from the excipient manufacturer, these steps are also critical for the drug producer’s independent risk analysis, as they make it possible to compare what both parties have observed.

Communicating TUP profiles
Preparing a TUP profile can take up to several months. And, although this might seem like a major investment, it ultimately pays off. As mentioned earlier, finding foreign matter can result in entire raw material lots being unnecessarily rejected by users.
Precious time, money and resources are wasted investigating particles that never posed a threat to patient safety and were technically unavoidable. Hence, the last and very important step regarding TUP profiles is communicating and collaborating with stakeholders. Establishing a standardised process to handle typical and atypical particles is highly recommended.
TUP profiles should always include information on the size, composition and expected number of particles.
Ideally, each profile should be linked to a flowchart of production processes. Including images of the particles in this documentation allows a quick comparison between TUPs and other particles found in the raw material lot — especially if they are very similar; this can yield significant time savings in connection with identification and analysis.
Moreover, information on solubility and the possibility of the particles’ removal, as well as known guideline limits, should be provided. As best practice examples, mitigation strategies should also be included when applicable.
Managing the unavoidable
TUP profiles play an important role in quality control. They foster a better understanding of chemical manufacturing and its limitations, while enabling pharmaceutical manufacturers to perform a quick and reliable risk assessment for direct materials and final products.
Moreover, they help to differentiate between issues that require attention and action and those that do not, which in turn reduces unnecessary actions and crisis management. Proactively managing and communicating TUPs can both mitigate risks and save valuable resources. Merck’s TUP reports for the Emprove portfolio provide a good example.
References
- IPEC, Technically Unavoidable Particle Profile (TUPP) Guide: https://ipec.org/sites/default/files/files/TUPP_GUIDE_2015.pdf (2015).
- A. Drakulich, “Atypical Visible Particles,” Pharmaceutical Technology 36(7), 86–88 (2012).
