A permeation enhancing technology developed by Apricus Bioscience and NexMed USA has recently been used in a drug approved by Health Canada. Richard Martin, Bassam Damaj and Dan Frank explain its use in optimising pharmacokinetics
The NexACT technology developed by NexMed consists of proprietary chemical entities that improve solubility and permeation of drugs through tissue and cell membranes resulting in enhanced drug permeation1,2 and/or oral absorption. These permeation enhancers temporarily change the permeation dynamics of the lipid bilayer and loosen the tight junctions between the cells, which enables an increased absorption of drug into the systemic circulation or an increase in local absorption (see Figures 1a & b).
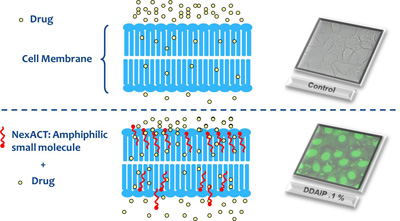
Fig. 1b: Mechanism of enhanced permeation. Real-time confocal microscopy of the DDAPI on uptake of Green Nucleic Acid Stain compared with control
The NexACT multi-route drug delivery platform consists of more than 100 different skin permeation enhancer molecules that could be used in drug formulation to increase permeation and achieve higher bioavailability.
