The pharmaceutical landscape has changed dramatically in just a few short years. Only a decade ago, the world’s top 10 selling medicines were all small molecules. Fast forward to 2014, the most recent year for which data are available, and it looks very different. Just half of the 10 biggest sellers were small molecules, joined by four monoclonal antibodies and a modified form of insulin.
With drugs for the ‘easier’ targets now widely available and many being adequately served with generic medicines, the process of new drug discovery has become more challenging, and pharma and biotech companies have had to become even more creative.
Biologics have developed into an important part of the fight against disease, and the challenges now facing players in the medicines market are finding ways of making safer, more efficacious drugs; exploring innovative combination therapies; and developing more efficient manufacturing processes. Catalent has developed a number of innovative technologies, and forged collaborations with similar thinking, pioneering companies to offer researchers more tools to create ‘better biologics’.
Multiple therapies within doses
Combination antibody therapies are gaining in importance in a variety of indications in oncology, immuno-oncology and infectious diseases. They can attack multiple targets at the same time, which may make them more effective in treating these diseases. However, as they contain multiple actives, they are even more expensive to make than a single antibody drug.
Catalent has undertaken a project with a third party under a licence agreement to use its own proprietary GPEx cell line engineering technology to improve the production capability of cells. The ability to create antibody combinations consistently and cost-effectively will be crucial if this type of complex antibody therapeutic is to succeed in the clinic, and will allow investigators to study the effects of combinations earlier in the development cycle.
Higher performance cell lines
Technologies such as Catalent’s GPEx cell line engineering offer faster access to reliable and productive cell lines. GPEx technology is based on a proprietary, pseudo-typed, high-performance vector that generates high-performance, highly stable, production cell lines in a wide variety of mammalian host cells, including; CHO-S, CHO-K1, CHO-DG44, HEK293, CAP and others. Virtually any cDNA (mAb and multigenic applications) can be packaged into the GPEx retrovector and used to transduce Catalent’s partners’ choice of mammalian cell line. These are extremely important when developing better biologics as they speed up the development time, and reduce the cost of production, allowing successful antibodies to reach patients more quickly and cheaply.
GPEx technology is already being used in the manufacture of five marketed products, including biosimilars, and many more antibodies and fusion proteins are under development
GPEx technology uses a retrovector technology that ensures the stable transduction of targeted cells, approaching 100% efficiency. This level of efficiency eliminates the requirement for selectable markers, and stable clonal cell lines are produced rapidly. GPEx technology utilises additional transductions to increase copy number or to add other complementary genes. More than 460 different mAb and mAb fusions, and 50+ different recombinant proteins have been produced using the GPEx system, achieving large scale fed-batch production titers of over 7.0g/L (CHO-S) and 2.5g/L (HEK293), making it a proven technology.
Stable cell lines are also important, and the technology has been demonstrated up to 100 generations. Development is fast, as stable pools of cells that produce the target proteins are generated from the outset, with no need for stability testing. GPEx technology is already being used in the manufacture of five marketed products, including biosimilars, and many more antibodies and fusion proteins are under development with multiple clients.
Next-generation antibody-drug conjugates
Antibody-drug conjugates (ADCs) have become de rigueur for pharmaceutical oncology drug development pipelines. There are presently more than 50 ADCs undergoing clinical trials, and many more in preclinical development. The field has rushed to follow the initial successes of Kadcyla (ado-trastuzumab emtansine, Genentech) and Adcetris (brentuximab vedotin, Seattle Genetics). Within the research realm, innovations in conjugation chemistry and linker technologies have suggested that there is much room for improvement in the conventional methods.
These combine the targeting and extended half-life properties of a monoclonal antibody with the cell-killing activity of a highly potent chemotherapeutic agent. The result is a more directed and, potentially, effective cancer treatment. ADCs are notoriously variable in structure, with first generation manufacturing techniques giving a wide distribution in terms of the number and location of API molecules attached to the antibody. Other issues include the inherent stability of the chemical linkers used to join the two components together, and poor production efficiency. All of these issues can affect the product’s efficacy, and increase costs.
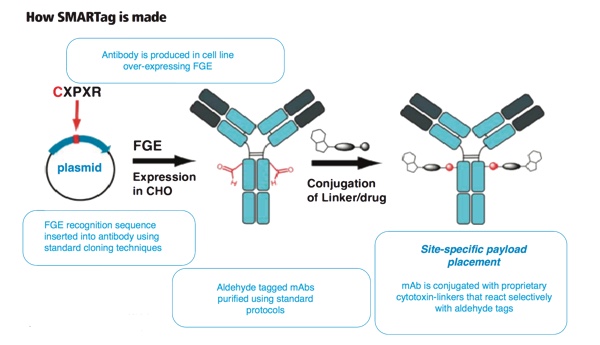
SMARTag technology allows a more uniform drug-antibody ratio to be achieved, offers the ability to direct the sites of attachment more accurately
The SMARTag technology, originally licensed from Redwood Bioscience before the company was acquired by Catalent in 2014, allows a more uniform drug-antibody ratio to be achieved, alongside an ability to direct the sites of attachment more accurately. By employing a naturally occurring enzyme to introduce a specific chemical group at directed points along the protein chain of the antibody, the number and location of the API molecules can be more carefully controlled. Only minimal cell line engineering is required.
The novel linker technology employed is more stable when travelling through the body to the site of action, and thus, the API is much less likely to be lost in transit. It also means that it is less likely to cause side-effects by damaging cells elsewhere. Importantly, the final ADC is much more homogeneous in nature than first generation products, which is important when satisfying the regulators that its efficacy and side-effect profiles will be reproducible. As ADCs continue to make up an increasing share of the oncology pipeline, there are significant opportunities to leverage recent advances in bioconjugation technology to create the next generation of differentiated ADC therapies to address unmet medical needs.
Better analytical techniques
In recent years, models for outsourcing analytical testing have changed. Traditionally, late phase routine programmes, and problem solving activities were kept in house. Today, contract labs are more commonly engaged during earlier product development phases and deal with some of the most challenging projects; working through problems together with the sponsor organisation.
Analytical testing plays an important role in all phases of biopharmaceutical development with new trends in the biotech industry driving innovation and creative outsourcing solutions. Analytical technology is also moving on at a pace, and is another important factor to be considered when developing better biologics. State-of-the-art techniques that prove the activity, stability and reproducibility of the biologics are essential if the increasingly strict demands of the regulators are to be met.
As the industry has matured, there are greater expectations for analytical detail to fully inform developers of a molecule’s characteristics
As the industry has matured, there are greater expectations for analytical detail to fully inform developers of a molecule’s characteristics, especially in the ever-advancing field of biosimilarity, and the requirements that are needed to prove equivalence to satisfy regulatory authorities. The recent licensing of the first biosimilars in the US – almost a decade after biosimilars reached the market in Europe – is likely to increase demand for analytical services, especially an assessment of biosimilar molecules that require an orthogonal analytical approach.
Any one particular analytical technique is not able to fully define the physical and biological characteristics of an original biotherapeutic or its potential biosimilar. Instead, a panel of analytical techniques must be used that can assess various and related, and sometimes overlapping characteristics of the molecule. This allows a more complete and integrated understanding of the biotherapeutic molecule’s form and function. This goal can be achieved by a combination of physicochemical analyses, such as mass spectrometry, size exclusion HPLC, SDS-electrophoresis (gel or capillary) isoelectric focusing (gel or capillary) and glycoanalysis, as well as more biological analyses such as potency (bioassay or ELISA) and binding assays (such as surface plasmon resonance or bio-layer interferometry).
This type of approach is the essence of ‘orthogonal’. Using an orthogonal approach, a more exact comparison may be made between a biotherapeutic molecule and its potential biosimilar.
GPEx and SMARTag are registered trademarks of Catalent.
The Authors
Michael Merges, Director, Biopharmaceutical Support Services; Rekha Patel, Director, Large Molecule Analytical Chemistry; Min Park, Group Product Manager, Advanced Drug Delivery Technologies, Catalent Pharma Solutions
