Bio-Rad Laboratories has released the first set in a new product line of PrecisonAb antibodies, rigorously validated for use in western blotting. These antibodies are designed to offer superior sensitivity, specificity and reliability. They also come with very complete validation data so that researchers can accurately assess the antibodies’ performance before buying, and a positive control lysate so that researchers can optimise the antibodies in their own labs.
These are the first of thousands of PrecisionsAb antibodies Bio-Rad plans to release to help researchers improve western blotting results.
Scientists have struggled with antibody unreliability for decades because manufacturing standards and quality control vary widely among vendors, targets, and even among antibody lots.
'There’s a huge discrepancy between the claims of antibody performance from vendors and what scientists encounter when the actual product arrives,' said Tiffani Manolis, marketing manager for protein quantitation at Bio-Rad. 'This discrepancy has created much distrust in the market. Our stringent validation and quality control ensures only top-performing antibodies are shipped along with extensive validation data. Customers can have confidence in their antibodies and their results.'
Bio-Rad’s PrecisionAb antibodies are screened using whole cell lysates from up to 12 different biologically relevant cell lines — by far the industry’s most rigorous validation process, Bio-Rad says. Only antibodies that detect endogenous protein levels with high sensitivity and specificity are included in the PrecisionAb product line. Each new lot is screened before release to ensure lot-to-lot reproducibility.
A worldwide trial, performed in a range of laboratory environments, confirmed the high specificity and sensitivity of the antibodies, says Bio-Rad.
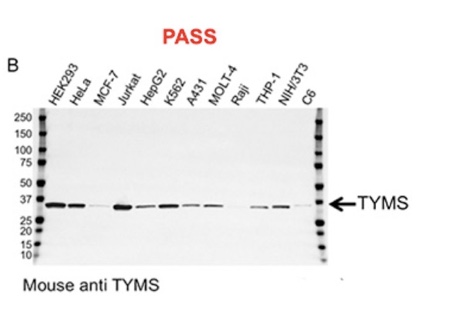
Validation data for two antibodies. A, this carbonic anhydrase IX (CA9) mouse monoclonal antibody failed validation due to nonspecific binding and low signal-to-noise ratio; B, this thymidylate synthase (TYMS) mouse monoclonal antibody passed validation showing high specificity and sensitivity.
'From what we have seen testing an initial range of PrecisionAb antibodies, the product performs well with little optimisation and at low concentration,' said Subhash Verma, PhD, an associate professor of molecular microbiology and immunology at the University of Nevada, Reno. 'Reliability is always a concern with antibodies, but Bio-Rad’s approach to quality control gives me considerable confidence that its products will stand apart from current offerings.'
According to Bio-Rad, the beta testers also valued the greater depth of information these antibodies provide. Bio-Rad shows the entire western blot image used for validation, giving the customer a complete view of antibody quality.
These antibodies also offer numerous benefits for optimising protocols and validating antibody specificity in a researcher’s sample, which include:
Trial sizes for all antibodies — enable researchers to test the antibody and optimise experimental conditions without a large upfront investment.
Detailed validation protocols — streamline optimisation and validation, saving time and reagents and delivering top results
Positive control lysates — provide researchers confidence in their data by simplifying validation of antibody specificity and troubleshooting of western blotting protocols.
For more information, please visit http://www.bio-rad.com/PrecisionAbpr.
