The production and study of liposomes is becoming an increasing field of review, due their biocompatibility, biodegradability, low toxicity, and ability to trap both hydrophilic and lipophilic drugs.1
Consisting of natural and synthetic phospholipids, liposomes are artificially prepared vesicles, commonly used as a cell mimic to study protein-protein interactions, protein-lipid interactions, drug delivery and encapsulation.2 The preparation of homogenous liposomes of various sizes allows the interpretation of membrane curvature, which plays a vital role in cell signalling, endocytosis, exocytosis, membrane fusion and protein trafficking, and so this process has many advantages.2
Liposomes can be classed via their size and lamellarity, as shown in Figure 1. It is these characteristics, along with lipid composition that determine the stability and encapsulation efficiency.
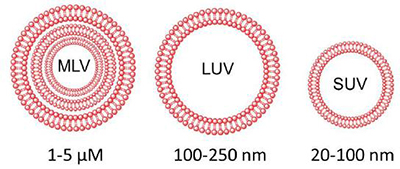
Figure 1: Classification of liposomes based on size and lamellarity.
Multilamellar vesicles (MLVs) are relatively unstable, and are not effective drug carriers due to the small core volume. Extrusion may be performed, whereby the MLVs are forced through polycarbonate filters with defined pore sizes. This disrupts the lipid bilayers of the vesicles, and if the force is strong enough, the MLV is completely torn apart, creating smaller fragments, which immediately form as small unilamellar vesicles (SUVs) or large unilamellar vesicles (LUVs) at the other side of the membrane, with a diameter reflecting the pore size. The resulting unilamellar vesicles are more stable, retaining high encapsulation capacity.
By performing a number of passes through the membrane, a particle size may further reduce, along with a narrower particle size distribution, as shown in Figure 2 (below).
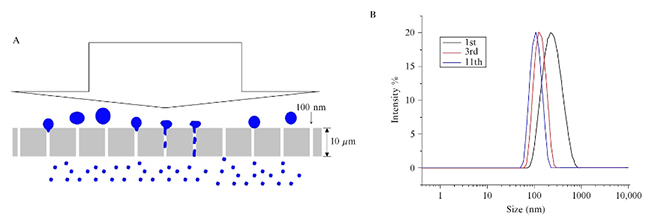
Figure 2. Forming unilamellar liposomes of the defined size by extrusion through a defined pore membrane filter. (A) Schematic representation of the extrusion process. (B) Graph reflecting uniformity of size via repeated passages of the liposomes through the membrane.
In addition, LUVs may be the desired product of extrusion, as they also show high encapsulation efficiency due to its large core volume. Producing these rather than SUVs would simply be a case of increasing the filter pore sizes in the extruder membranes.
Avestin’s EmulsiFlex range of high pressure homogenisers allows operators to perform both homogenisation and extrusion of liposomes in solution. Each model facilitates controlled, consistent homogenising pressures, to narrow the distribution of liposome sizes once forced through the filters. In addition, Avestin instruments account for the combination of homogenisation and extrusion in a continuous process, which greatly reduces the number of required passes to achieve a desired particle size distribution. This is one of the reasons why extrusion has become a popular, time-effective, and easily reproducible technology, when considering unilamellar liposome formation, with no detectable degradation of phospholipids.
Other methods of liposome formation are available; however they do have increased limitations. For example, one disadvantage of sonication for liposome extrusion, is the process resulting in “limit size” vesicles, which are subject to lipid packing constraints, lipid degradation and heavy metal contamination.3 Sonication, along with other methods such as freeze-thaw and sedimentation, have limitations regarding the consistency and efficiency of liposome preparation with a highly curved surface (i.e diameter <100nm). This limits the use of these liposomes for membrane curvature sensing.2 Moreover, reverse phase evaporation and detergent dialysis can produce large unilamellar liposomes (LUVs); however this method involves the practical difficulties of lipid solubility and complete removal of detergent, respectively.
Finally, when considering scalability, Avestin not only offer EmulsiFlex benchtop homogenisation/extrusion systems, but also pilot and production scale, so whatever level of product manufacturing you may be at, Biopharma’s Avestin equipment range can offer the solution.
For more information on the Avestin series, visit www.biopharma.co.uk or contact Ashley Morgan (BSc) on amorgan@biopharma.co.uk | +44 (0)1962 841092
References
- Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Woo Joo, S., Zarghami, N., Hanifehpour, Y., Samiei, M., Kouhi, M. and Nejati-Koshki, K. (2013). Liposome: classification, preparation, and applications. Nanoscale Research Letters. 8 (1), 102.
- Hope, M.J., Nayar, R., Mayer, L.D. and Cullis, P.R. (1993). Reduction of Liposome Size and Preparation of Unilamellar Vesicles by Extrusion Techniques. Liposome Technology (G. Gregoriadis, Ed). 1, 123.
- Morton, L.A., Saludes, J.P. and Yin, H. (2012). Constant pressure-controlled extrusion method for the preparation of Nano-sized lipid vesicles. Journal of Visualized Experiments. (64), pii 4151.

