Increasingly, industry has become driven by more stringent regulations, where companies are asked to understand specific material properties, of intrinsic value during the design and implementation of an efficient lyophilisation recipe. Thorough awareness of these critical parameters then helps ensure successful drying with minimal impact, if any, on overall product efficacy.
Equally, advanced techniques to determine other key aspects, such as residual moisture content, both post and real-time during the cycle, are being actively promoted and utilised to such an extent that these are now of paramount importance for pilot and production scale freeze drying processing.
In light of this trend, for R&D scientists and companies alike, there is greater emphasis on designing the ‘perfect cycle’, which not only dries the product in an efficient and commercially viable manner but does so to a reproducible end moisture content, specified by the user ‘in question’.
For most materials, a lower residual moisture content post drying will increase the product's shelf life. This obviously allows the sample to remain on the shelf for longer before it must be discarded – therefore enhancing overall company profits. Previously, users would wait until the run was complete before performing a post-drying Karl Fischer Titration, for example.
The SP Scientific/Biopharma range of freeze dryers have, however, reacted to this demand, allowing users a choice of end point determination methods, in-situ of the dryer, to establish residual moisture levels of their material during the run itself. These techniques have been described below in a little more depth, ranging from standard testing protocols, towards the cutting edge of freeze drying determination technology.
Pressure rise test
This technique relies on the inclusion of a point of isolation between the product chamber and condenser, generally through the presence of a butterfly valve. The PLC based Allen Bradley software available on the SP Scientific Genesis/Ultra systems, provides added functionality, written in to a user’s specific recipe, such as:
- Barometric end point process control software
- Pressure rise test for product dryness
- Automatically closes/opens isolation valve
- Measures vapour pressure rise in product chamber
- Includes option to remain in current step until user defined pressure rise criteria is met
- The user can then carry out calibration studies to relate the pressure rise test results, to residual moisture content in the product
Pirani vacuum sensor/capacitance manometer feedback
Comparison of both the Pirani gauge and capacitance manometer can be carried out as a visual/manual inspection of vacuum readings to determine completion of primary drying – this is due to the Pirani consisting of a hot wire gauge, influenced approximately between +/- 15% in the presence of gases, such as water vapour.
The capacitance manometer, however, will generally record an absolute measurement, with minimal overage i.e. approximately +/- 0.15%.
Taking this knowledge into account, the point at which both sensors read the same value, we can, with a degree of confidence, ascertain the product has dried, because the Pirani sensor must no longer be ’skewed’ by the presence of water vapour – the sublimation process would appear to have ceased.
However, the SP Scientific/Biopharma machines go a step further, by using the Pirani/Capacitance Manometer Differential Automated Process Control software. This, again, enables the equipment to remain in its current step until the user defined pressure differential is met, before automatically advancing to the next stage.
User recipes, and their advancement through particular steps can therefore be determined based on this feedback loop, and specific understanding of residual moisture content for each defined step.
Sample thief/extractor
Another popular feature on the SP Scientific Genesis freeze dryer is the ability to remove individual vials, mid-cycle, to allow for external moisture analysis, such as Karl Fischer Titration, to be carried out. The configuration comes complete with removable jaws/arm to remove specific vials, in conjunction with a separate vacuum pump, for evacuation of the sample chamber, where the container can be stoppered under a controlled vacuum.
This has become a favoured accessory, making the SP Scientific pilot level Genesis an extremely popular R&D/pilot scale tool, because technicians are now able to conduct analysis mid run on individual vials, without compromising the batch.
Tunable diode laser adsorption spectroscopy
This is one of the most advanced and exciting contemporary technologies in the area of freeze drying, where research scientists have already begun to adopt the method as their ‘desired’ end point determination tool. SP Scientific/Biopharma Group have answered this call, through the cutting edge Lyostar3, where the technology of TDLAS has now become exclusive to this manufacturer.
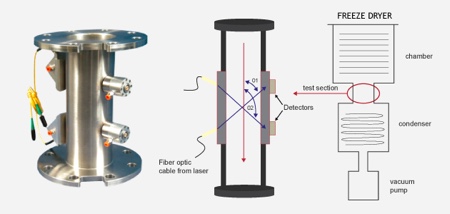
Tunable Diode Laser Absorption Spectroscopy has the ability to improve process performance understanding, through the use of non-intrusive detection
The benefit of tunable diode laser absorption spectroscopy is the ability to improve process performance understanding, through the use of non-intrusive detection methods. These consist of water vapour presence, flow velocity, and water mass flow (dm/dt) measurements.
In addition, and unlike other methods of identification, TDLAS will measure the gram weight of vapour sublimed at a specific point of the process – information R&D scientists and pharmaceutical corporations are taking advantage of more and more.
For more details on the SP Scientific range of research/scale-up systems, including the Lyostar3, please contact Richard Lewis: lewis@biopharma.co.uk or +44 1962 841092.

