Epidiolex is a cannabis-derived CBD (cannabidiol) oral solution prescribed for the treatment of refractory epilepsies, whereas Sativex, which contains both THC and CBD, is approved for the symptomatic relief of spasticity in adult patients with multiple sclerosis.
Both the FDA and EFSA have cited potential liver damage as a possible side-effect regarding CBD as a supplement and as an ingredient in food and drinks.
But is CBD really that toxic to the liver? Here, Cristelle Santos, Toxicology Consultant at Broughton, takes a closer look at the scientific evidence.
CBD and liver function
CBD as a chemical entity is highly purified and relatively well tolerated. However, there are some important caveats to investigate in more detail, one of which is liver function.
CBD is metabolised in the liver, primarily by enzymic hydroxylation (CYP3A4 and CYP2C19) to an intermediate product, 7-OH-CBD, which is then metabolised further and excreted in faeces and urine.1
The main plasma metabolites of CBD in humans are understood to be 7-COOHCBD and 7-OH-CBD, with plasma concentrations of up to 47-fold and 1.2-fold higher, respectively, than the plasma concentration of CBD.2
In addition to CYP3A4 and CYP2C19, seven other human CYP enzymes have been identified as capable of metabolising CBD: CYP1A1, CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4 and CYP3A5 (Figure 1).3
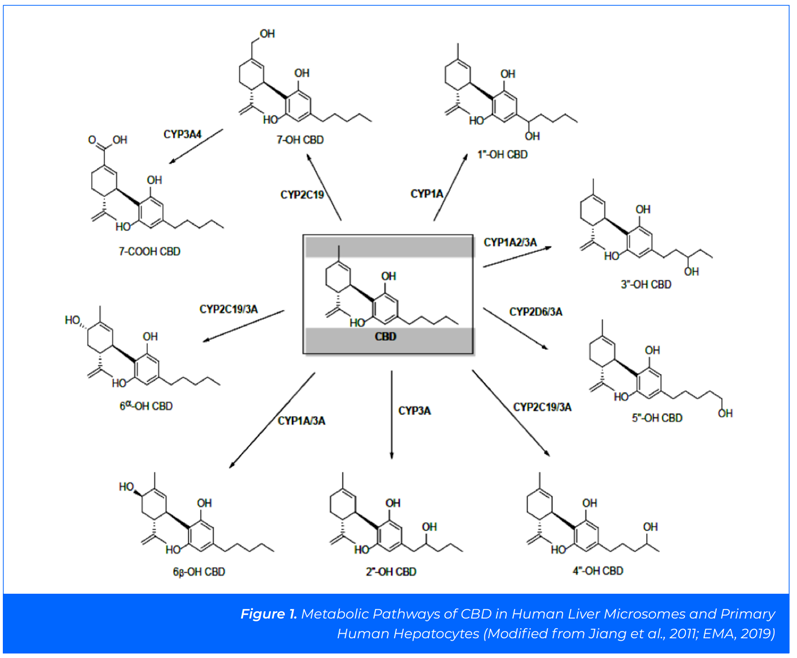
The toxicity potential of CBD has been investigated in clinical and non-clinical studies. Several of these studies were conducted to support the registration of Epidiolex and details of these studies are publicly available.
The toxicity concern arose from results indicating that some children developed liver problems following Epidiolex use. Specifically, an increase in liver enzymes was noted, indicating that the organ may be inflamed or damaged.
Reviewing the scientific evidence
Currently, the UK Food Standards Agency (FSA) recommends a daily limit of 70 mg of CBD/day (1 mg/kg bw/day) based on the Epidiolex submission — wherein no effect on levels of the liver enzyme alanine aminotransferase (ALT) were observed with doses of less than 5 mg/ kg bw/day.
Therefore, the FSA concluded that 1 mg/kg bw/day is unlikely to result in drug-drug interactions.4 More recent clinical reviews have been used by the Australian Therapeutic Goods Administration (TGA) to support daily doses of up to 150 mg/day of CBD.5–8
In 2020, the Chesney group analysed 12 clinical trials involving 803 participants and noted that liver issues occurred in children using both Epidiolex and another antiepilepsy drug. Antiepileptic drugs are known to cause liver enzyme elevations.
The authors concluded that “CBD is well tolerated and has few adverse effects. However, its ability to inhibit the hepatic metabolism of other medications (such as clobazam and sodium valproate) has the potential to cause adverse effects.
This is demonstrated by the increased rates of adverse events, serious adverse events and withdrawals in trials for childhood epilepsy syndromes but no other indications.”
Therefore, it is evident that although CBD may cause some liver function elevations, the elevation is derived from association with other drugs in most cases.
The Dos Santos group reviewed 18 randomised controlled trials (RCTs) involving a total of 1127 subjects. The authors concluded that “no serious adverse effects (SAEs) were observed in the studies with healthy volunteers.
A higher incidence of SAEs was observed in the RCTs with epilepsy. Severe somnolence, lethargy, increase in hepatic transaminases, rash and pneumonia accompanied by respiratory failure were some of the most relevant effects observed.
Notably, these seem to be related to drug–drug interactions, particularly with valproate in the case of increases in hepatic transaminases and with clobazam in the case of somnolence, lethargy, rash and pneumonia accompanied with respiratory failure.”
Numerous publicly available clinical studies have evaluated the pharmacokinetics, efficacy and safety of CBD at doses of up to 1500 mg/day (the dose required to treat epilepsy).
EFSA reviewed several animal studies that reported that very high doses of CBD may damage the liver.9 In terms of human data, liver toxicity has also been observed at high levels in various studies (Table I).
Table I shows that the dosage in the studies presented is generally considerably higher than the limits recommended by the UK FSA and the Australian TGA. Studies that used a phase-in period to accustom patients to CBD reported lower incidences of increased transaminases than those observed in other studies.10
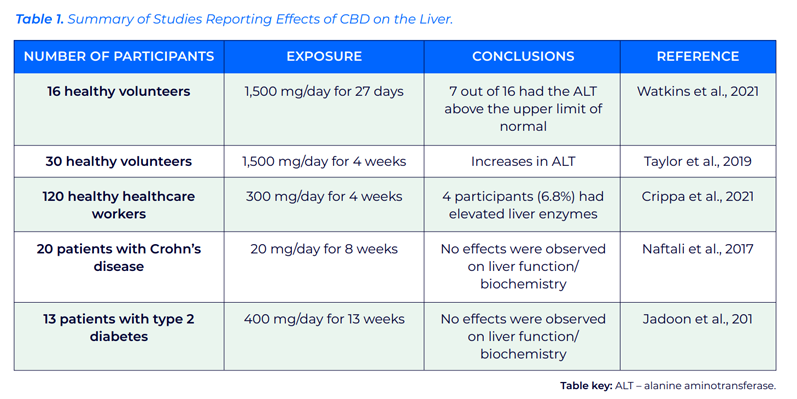
EFSA reviewed several studies investigating liver enzyme and bilirubin levels in children and adolescents with epilepsy that had been treated with Epidiolex and clobazam or valproate.
An increase in ALT or aspartate aminotransferase (AST) was reported in all studies at all dose levels. However, as stated above, this is expected to result from drug-drug interactions or an effect of the antiepileptic medication.
EFSA concluded that studies in healthy volunteers show increases in liver and pancreatic enzymes (ALT, AST, alkaline phosphatase [ALP] and gamma-glutamyl transferase [GGT]) at Epidiolex doses of 20 mg/kg bw/day or up to 1400 mg/day assuming a 70 kg adult body weight).
This is 20 times higher than the 70 mg/day maximum daily recommended dosage by the UK FSA and nine times more than the 150 mg/day Australian TGA recommended daily intake. Recent research shows that CBD does not cause hepatotoxicity in humans at low doses.
Kaufmann et al. published a study involving approximately 800 people consuming CBD. Participants consumed CBD for a minimum of 30 days before the liver tests were performed and no alterations in liver enzymes were observed.11 The authors concluded that self-medication of CBD is not associated with damage to the liver.
A further study published by the same research group observed 1061 adults during their usual CBD regimen for at least 30 days. The authors concluded that there was no relationship between the results of the liver tests and CBD dose and that “self-dosing CBD was not associated with an increased prevalence of elevation of liver tests.”12
In June 2023, the first 14- and 90-day toxicity studies were published, filling a very important gap in the safety profile of CBD.
In these 14-day subacute and 90- day subchronic toxicity studies, the administration of CBD at concentrations up to 150 and 140 mg/kg bw/day, respectively, by oral gavage did not produce any significant toxic effects. The authors reported that CBD was well tolerated at these doses.13
The importance of understanding dosage levels
Based on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Technical Guidance Document (TGD), equation 1 (below) is appropriate for the calculation of a derived no-effect level (DNEL).14

The no observed adverse effect level (NOAEL) of 140 mg/kg bw/day from the 90-day study in male and female Sprague Dawley rats was selected as the highest dose reporting no significant effects.
The NOAEL, assuming an adult body weight of 70 kg, was divided by the total number of relevant assessment factors (Table II) to give a DNEL of 49 mg/day.
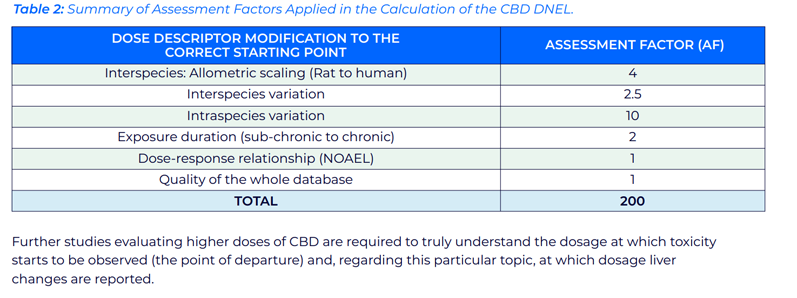
Further studies evaluating higher doses of CBD are required to truly understand the dosage at which toxicity starts to be observed (the point of departure) and at which dosage liver changes are reported.
Summary
In summary, although some evidence suggests a potential risk of liver damage associated with CBD use, it appears to be relatively rare and more likely to occur at high doses or in combination with other drugs.
So, the best way to answer our initial question about whether CBD really does harm the liver is to ask Paracelsus, the father of toxicology.
I’m sure his answer would be: “The dose makes the poison,” indicating that current scientific evidence indicates that a greater understanding of dosage levels and other contributing factors to adverse reactions is key to safeguarding public health and paving the way for the full potential of the therapeutic benefits of CBD to be realised without compromising safety.
References
- G. Hawksworth and K. McArdle, “Metabolism and Pharmacokinetics of Cannabinoids,” in G.W. Guy, B.A. Whittle and P.J. Robson, Eds., The Medicinal Uses of Cannabis and Cannabinoids (Pharmaceutical Press, London, UK, 2004) pp 205–228.
- https://doi.org/10.1007/s40263-018-0578-5.
- R. Jiang, et al., “Identification of Cytochrome P450 Enzymes Responsible for Metabolism of Cannabidiol by Human Liver Microsomes,” Life Sci. 89(5-6), 165–170 (2011).
- www.food.gov.uk/news- alerts/news/food-standards-agency-sets-deadline-for-the-cbd- industry-and-provides-safety-advice-to-consumers.
- E. Chesney, et al., “Adverse Effects of Cannabidiol: A Systematic Review and Meta-Analysis of Randomized Clinical Trials,” Neuropsychopharmacology 45(11), 1799–1806 (2020).
- C. Larsen and J. Shahinas, “Dosage, Efficacy and Safety of Cannabidiol Administration in Adults: A Systematic Review of Human Trials,” J. Clin. Med. Res. 12(3), 129–141 (2020).
- R.G. Dos Santos, et al., “Serious Adverse Effects of Cannabidiol (CBD): A Review of Randomized Controlled Trials,” Expert Opin. Drug Metab. Toxicol. 16(6), 517–526 (2020).
- www.tga.gov. au/news/media-releases/over-counter-access-low-dose- cannabidiol.
- https://doi.org/10.2903/j.efsa.2022.7322.
- G. D'Onofrio, et al., “Slow Titration of Cannabidiol Add-On in Drug-Resistant Epilepsies Can Improve Safety with Maintained Efficacy in an Open-Label Study,” Front. Neurol. 11, 829 (2020).
- R. Kaufmann, et al., “Observed Impact of Long-Term Consumption of Oral Cannabidiol on Liver Function in Healthy Adults,” Cannabis Cannabinoid Res. 8(1), 148–154 (2022).
- R. Kaufmann, et al., “The Effects of Long-Term Self- Dosing of Cannabidiol on Drowsiness, Testosterone Levels and Liver Function,” Med. Cannabis Cannabinoids 6(1), 32–40 (2022).
- R.G. Henderson, et al., “Oral Toxicity Evaluation of Cannabidiol,” Food and Chemical Toxicology 176, 113778 (2023).
- https://echa.europa.eu/documents/10162/13632/information_ requirements_r8_en.pdf/e153243a-03f0-44c5-8808- 88af66223258.
