The pharmaceutical industry has changed dramatically, notes Klaus Ullherr, Senior Product Manager at Syntegon Technology. Just ten years ago, blockbusters and traditional drugs produced in large batches were the focus of many research and manufacturing companies.
Today, these medicines continue to make an important contribution to human health. Some of them, such as blood thinners, painkillers and insulin, have become an indispensable part of the drugstore shelf.
They are produced on lines in 24-hour shifts, some of them 30 m long, and, thanks to sophisticated process and packaging technologies, their continued production is assured for the foreseeable future.
From blockbusters to ATMPs
At the same time, increasingly individualised therapeutic approaches to the treatment of diseases requires a change of thinking. Special and often personalised medications are now available to cure or at least delay some life-threatening illnesses.
These usually very costly drugs require a high level of commitment in research and development — as well as novel machine concepts that can process small and very small batches in a flexible way while facing changing requirements.
Cellular and molecular biotechnology has been accompanied by a revolution in pharmaceutical manufacturing: whereas high-speed, high-throughput lines used to be in great demand, more and more pharmaceutical companies are now investing in the development and commercialisation of small-volume medicines, which have very different production and filling requirements.
The increasing prevalence of advanced therapy medicinal products (ATMPs), such as cell and gene therapies and bioengineered tissue products, shows that the industry is specialising at pace. These drugs are not manufactured on huge lines. Instead, the focus is on what is commonly known as small batch production.
A precise definition of “small batch” is not specified in the ICH guidelines. The US FDA, however, provides an answer for generic parenterals.

According to “Guidance for Industry, ANDAs: Stability Testing of Drug Substances and Products, Questions and Answers,” small batches consist of at least 10% of the proposed maximum commercial batch size, or less than 15,000–60,000 containers based on fill volumes.1
The batch size shrinks to a few thousand containers for parenterals used in clinical trials. For highly specialiaed treatments such as autologous cell therapy, batches are even smaller. On average, a patient needs about 5–10 containers.
The highest flexibility at the lowest possible reject rates
The growing number of cell and gene therapies in the development phase underlines the importance of ATMPs. Conventional high-speed machines can no longer meet the requirements of these new products.
But how to produce these small or even very small batches economically? What must a line do to satisfy both pharmaceutical companies and patients? Flexibility is at the top of the list of requirements.
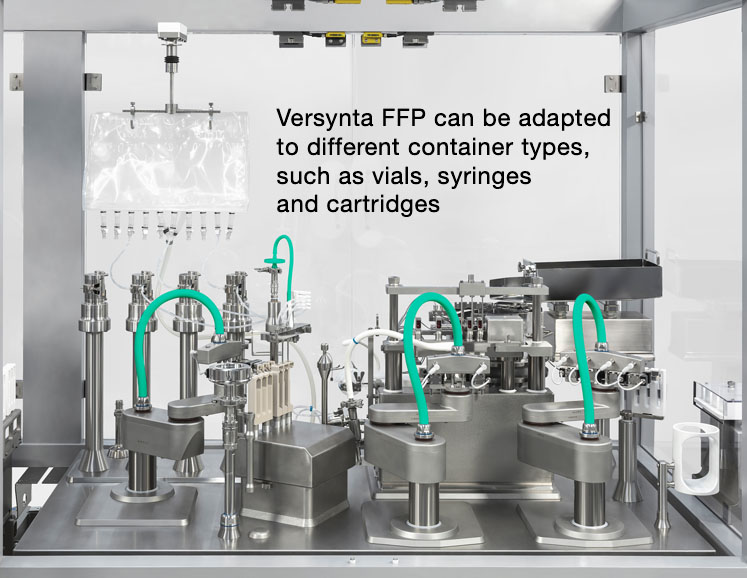
A wide variety of products must be filled into different container sizes and types such as vials, syringes or cartridges. Single-use technologies are particularly popular when it comes handling and processing these biotech drugs in a flexible manner as they eliminate the effort and costs involved in cleaning validation.
Another criterion for flexibility is the reduced number of format parts, which obviates the need for lengthy changeovers. In addition, these very small quantities require maximum product yield or low rejects. In a nutshell, any product loss must be avoided.
Automation for even higher safety
Human intervention is still the main reason for contaminated pharmaceuticals and automation is essential to reduce or eliminate this. For example, as far back as 2004, the FDA required that “the design of equipment used in aseptic processing should limit the number and complexity of aseptic interventions by personnel.”
In addition, the FDA states that “automation of other process steps, including the use of technologies such as robotics, can further reduce risk to the product.”2 In the long run, aseptic filling will evolve from human-centred to fully automated production with the use of appropriate technologies.
This change is already in full swing. For example, if containers within the isolator are conveyed to the filling station and on to the crimping station by a robotic arm, the risk of contamination is reduced manifold.

Robots can also decrease the number of format parts and eliminate any glass-to-glass contact. Newly developed systems, such as Syntegon's Versynta FFP (Flexible Filling Platform), also feature a laminar flow-optimised design to make sure that the air flow can reach the containers and flow around them without obstruction.
One hundred per cent in-process control (IPC) during the filling process reduces product loss to a minimum and ensures that almost every millilitre of the high-quality product is filled.
Gloveless production of micro batches
Following the small batch principle described above, the process goes even further — in a numerically downward way. Although a modular small batch system such as the Versynta FFP can handle up to 3600 vials, syringes or cartridges per hour, its little sister processes a mere 120–500 containers per hour.
The highly flexible, fully automated Versynta microBatch production cell fills and seals the smallest batches in different containers. Batch changes are possible in only 2 hours. Syringes, cartridges and vials made of glass or plastic can be filled with virtually no product loss.
The dimensions of the machine are just as small as its output. With a length of barely 3.5 m, a width of approximately 2 m and a height of 3 m, the machine can easily be integrated into existing production environments.
The isolator cell itself measures just 1.6 x 1.5 m. It houses the tub opening, the filling station (including 100% in-process control) and the combined stoppering and crimping station.
Thanks to the integrated air treatment system, hardly any interfaces to the building or technical ceiling installations are required. The new development sets new standards, particularly in terms of automation.
The gloveless isolator with integrated air treatment significantly reduces the risk of contamination by eliminating manual intervention on the part of the operating personnel. The microBatch set-up meets the highest sterility requirements as specified by Annex 1. Steam-sterilised parts are fed via port systems and installed by the robot.
RTU containers on the rise
The latest developments in the field of small and micro batches pay tribute to another trend. The market for ready-to-use (RTU) containers has been growing rapidly for years.
This is not surprising, as pharmaceutical manufacturing companies benefit from simpler processing procedures, reduced total cost of ownership and greater flexibility. RTU containers are the preferred choice for small batch production and their number is growing steadily.
According to a recent report, the market for RTU vials alone is expected to generate global sales of approximately $1.18 billion during the next 10 years, with an estimated growth rate of 14.5%.3
However, in addition to vials, presterilised cartridges are also becoming increasingly popular. First and foremost, however, RTU syringes paved the way for other presterilised containers as early as the 1980s.
In fact, the advantages of smaller batches of RTU containers are enormous. Bulk syringes, for example, are supplied non-sterile. As they are not stable-standing, they require complex handling in the machine and filling process.
Syringes also require siliconisation, which involves a special format-dependent process. The latter depends on the way the syringes are filled. Biotechnologically produced drugs, for example, require a particularly low level of siliconisation.
Savings in time, space and costs
The advantages of RTU containers become particularly clear in the process steps described above. Although they are currently still quite cost-intensive, they save pharmaceutical manufacturers a great deal of time, space and money.
Numerous steps, such as cleaning, siliconisation and sterilisation are outsourced to the packaging suppliers of RTU containers. They have the expertise and make sure that all processes are qualified and validated according to current global requirements and that containers are delivered with a certified endotoxin, germ and particle concentration. In the case of presterilised syringe systems, this also includes the required siliconisation process.
The demand for high-priced ATMPs and the variety of RTU packaging materials are both rising steadily — as are the requirements for new equipment for small and very small batch sizes.
The latter should not only be flexible in the processing of the packaging materials; appropriate machines must also meet the trends in terms of automation and the least possible human intervention.
In the best-case scenario, primary packaging material and machine manufacturers work closely together to develop new solutions.
Systems that ensure flawless processing for pharmaceutical manufacturers and the highest level of product safety for patients can only be developed if both partners not only react to trends, but proactively shape them together from the outset.
References
- www.fda.gov/files/drugs/published/ANDAs--Stability-Testing-of-Drug-Substances-and-Products--Questions-and-Answers.pdf.
- www.fda.gov/media/71026/download.
- www.psmarketresearch.com/market-analysis/rtf-rtu-vials-market-trends.
