An orphan drug is one that’s developed specifically to treat a rare disease, which is defined as a condition that affects fewer than 200,000 people in the US or one in 2000 in Europe.1
It’s estimated that there are more than 7000 rare diseases affecting 300 million people around the world.2 With only 5% of rare diseases having an approved treatment , there is clearly a large unmet medical need.
Recent developments, however, are enabling more orphan drugs to be approved than ever before, providing life-saving therapies to patients across the globe. With growing importance worldwide, it is predicted that orphan drugs will make up a fifth of all prescription drug sales by 2026 and almost a third of the global drug pipeline’s value.3
There is a vast variety of rare diseases; 72% are identified as being genetic whereas others are infectious forms, autoimmune diseases and rare cancers.3

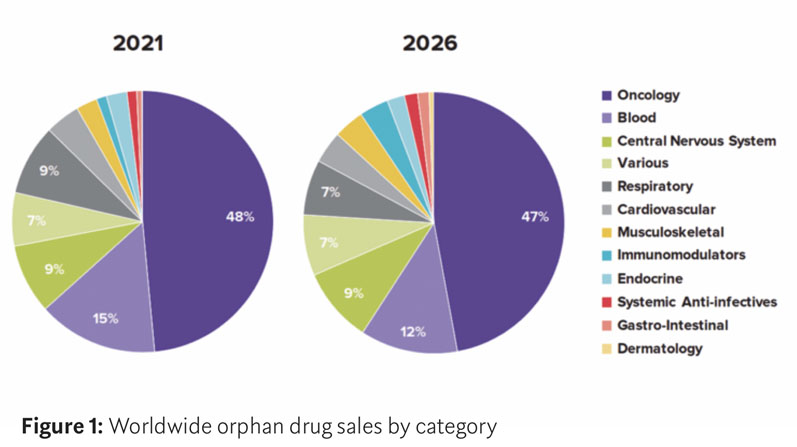
In a recent report by Evaluate Ltd, cancer remains the dominant therapy area for orphan drugs — accounting for six of the top ten pipeline candidates — with therapies for blood diseases and central nervous system diseases following (Figure 1).
Evaluate predicts that oncology orphan drug sales will grow by 70% between 2021 and 2026 and that, by 2026, cancer therapy orphan drug sales will have a combined net present value (NPV) of $29.6 billion.4
Even with economic and regulatory incentives, developing orphan drug formulations presents several challenges. Achieving clinical and commercial success requires biopharmaceutical companies to overcome a number of development and manufacturing challenges related to fast-tracked timelines, the high value and limited availability of active pharmaceutical ingredients (APIs), formulation and scale-up.
Addressing these challenges requires experience, expertise and scalable technologies, along with a flexible and agile supply chain.
Accelerated solutions
Driven by the need to provide life-saving therapies for an unmet clinical need and to take advantage of the streamlined review processes provided by the EMA Accelerated Approval pathway or, in the US, Fast Track, Priority Review, Breakthrough Therapy or Accelerated Approval, orphan drug products usually benefit from accelerated timelines.
For those orphan drugs that are granted an expedited approval pathway, the challenge for the biopharmaceutical company is to leverage their own or their partnering CDMO’s drug development knowledge and build a strategy that maintains the integrity, quality and timeliness of the manufacturing process, ensuring a complete CMC package is generated to support regulatory filings.
Aligning development, analytical, manufacturing and packaging functions internally or via a CDMO that can provide integrated end-to-end drug product solutions is vital to expedite time-to-patient and keep CMC activities on the critical path for regulatory submission.
Development and manufacturing efficiencies
The high value and limited quantity of orphan drug materials (drug substance and drug product) pose challenges throughout the drug product development lifecycle, from formulation and analytical method development through to clinical and commercial manufacture.
As a result, true efficiency is a priority when developing these orphan drug products. Early stage development must be process-orientated and data-driven to make the most of limited resources and derisk development programmes.
Employing Quality by Design (QbD) and Design of Experiment (DoE) approaches throughout the formulation and/or lyophilisation cycle development process aids in the identification and resolution of challenges such as solubility or bioavailability prior to scale-up, validation and manufacturing.
This helps to develop formulations and analytical methods that are robust, reliable and simplify the transition to clinical and commercial manufacturing. Utilising manufacturing processes such as drug in capsule (DIC) and fully automated sterile fill-finish help to preserve APIs and minimise line losses, as well as provide valuable cost and product efficiencies.
Considering that many orphan drug bulk drug substances are biological in nature, they can be difficult to come by and are produced in small batch sizes.
Maximising batch yields is of primary importance and tools such as filter and tubing size selection, QC, analytical and microbiological testing strategies and the use of fill equipment with non-destructive weight checks may be helpful.
Often, specialised sterile fill-finish approaches — such as using the Cytiva SA25 robotic platform — can provide tailored solutions in terms of high-quality primary packaging components (vials, prefilled syringes or cartridges) in smaller packaging sizes and with less product loss.
Flexible scale manufacturing
The lower patient populations associated with orphan drug products result in both smaller clinical batch sizes and less commercial batch manufacturing compared with most other drug formulations.
In addition, orphan APIs are typically complex molecules, such as cell and gene therapies or biologics that require specialised and flexible processing technologies to manufacture the drug product in relatively small campaigns — even for commercially approved orphan drugs.

Often, the use of disposable filling solutions provides complete manufacturing flexibility, maximising product yield while minimising any potential risk of cross-contamination at the manufacturing site.
Although developing a phase-appropriate formulation may be viable and the quickest solution in early stage trials, forethought and collaboration between development and manufacturing teams is essential to address any potential risks during scale-up and process validation.
If partnering with a CDMO, integrated drug product development, analytical and manufacturing solutions are vital to ensure scientific continuity and aid scale-up.
Simplified supply chains
Recent events have put a spotlight on the need for nimble and agile supply chains to ensure the supply of life-changing orphan drug therapies to patients.
The application of technologies such as late stage customisation (LSC) and just-in-time (JIT) supply models afford biopharmaceutical companies a much more streamlined approach; optimising valuable drug product inventory whilst minimising waste and loss ensures that vital medicines are delivered to patients around the world when needed.
Likewise, developing an effective lyophilised presentation of the drug product could simplify its overall supply chain and obviate the complexities associated with cold chain packaging, storage and distribution.
Security of supply
In an effort to address disruptions in manufacturing that cause drug shortages, and to ensure an uninterrupted supply of critical life-changing medicines such as orphan drugs, the US FDA has developed draft guidelines — Risk Management Plans to Mitigate the Potential for Drug Shortages — that help drug manufacturers to develop, implement and maintain risk management plans.
One risk control measure noted in the FDA guidelines is the identification of alternative/ back-up suppliers. Dual sourcing allows biopharmaceutical companies to appoint a second supplier to ensure that they have continuity of supply for their life-changing drug products and to help mitigate risk should something untoward happen to their primary supply source.
Dual sourcing can also provide the logistical agility and flexibility to deliver any additional supply in the occurrence of approval for a new indication.
The role of CDMOs
Specialist contract development and manufacturing organisations (CDMOs) have a key role to play by helping biopharmaceutical companies to navigate the development complexities and overcome the manufacturing challenges posed by small-volume orphan drugs.
Experienced outsourcing partners with expert technical capabilities and know-how can safely accelerate drug development through the clinical lifecycle towards commercialisation.
By working with a global CDMO that’s able to offer integrated solutions — from regulatory consultancy, formulation development, scalable manufacturing and packaging — biopharmaceutical companies can derisk their orphan drug programmes, saving time and reducing costs while ensuring that these important life-changing therapies reach patients when needed.
References
- www.orpha.net/consor/cgi-bin/Education_AboutOrphanDrugs.php?lng=EN
- www.rarediseaseday.org.
- www.nature.com/articles/s41431-019-0508-0.
- www.evaluate.com/thought-leadership/pharma/orphan-drug-2022-report.

