Jet milling is a simple and cost-effective way to make powders with a tight range of particle sizes. This is particularly important for drug delivery via inhalation, wherein the therapeutic effect is highly dependent on the drug’s ability to target specific regions of the lungs — which is partially controlled by tailoring the particle size.
For instance, delivery varies significantly for particles with diameters of 2–4 µm, whereas particles larger than the optimal maximum aerodynamic size of 5 µm are not effectively deposited in the deep lung.
An appropriate development strategy should be implemented to ensure process robustness and to derisk scale-up and industrialisation. The quality by design (QbD) approach can be used to understand the correlation between jet milling process parameters (such as grinding pressure and feeding rate) and product quality attributes.
This will inform successful scale-up and process control.
Nilotinib is primarily used to treat chronic myelogenous leukaemia, but it can also prevent airway inflammation and remodelling after chronic allergen challenges.
It therefore may have potential in severe chronic asthma.1 We developed a jet milling process to create nilotinib particles that were less than 5 µm following the strategy illustrated in Figure 1.
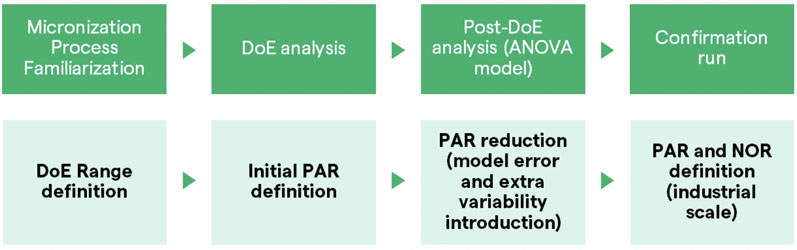
Figure 1: Schematic evaluation of jet milling process development
Micronisation process familiarisation
Less than 5 g of material was used for process familiarisation, taking a trial-and-error approach. The main goal was to make a preliminary assessment of the mill’s micronisation efficiency on nilotinib and then decide the range of conditions for the subsequent design of experiments (DoE).
For that, the specific energy of the process was varied from 2500–10,000 J/g, resulting in particles within target.
Additionally, solid state analysis is crucial; changes in this parameter can negatively affect the particles’ stability and how they interact with each other when formulated for inhalation delivery. In this case, solid state analysis confirmed that the particles did not amorphise during micronisation for the tested conditions.
Based on these results, process parameters were selected targeting a larger particle size distribution (PSD) and, thus, a reduced specific energy (from 168 to 1502 J/g): a feeding rate range of 100–300 g/h and a grinding pressure from 1–3 bar was used, with the central point having an energy of 501J/g.
DOE analysis and feasibility study
Following the familiarisation phase, a face-centred central composite DoE was used to investigate the micronisation process in a total of 28 trials (Figure 2).
The statistical evaluation of the DoE was performed using analysis of variance (ANOVA), with a power transformation function to ensure that residuals were normally distributed (looking at the Dv90 results).
A significant model was obtained (R2 of 0.85) and is represented by the 2D and 3D contour plots in Figure 2.
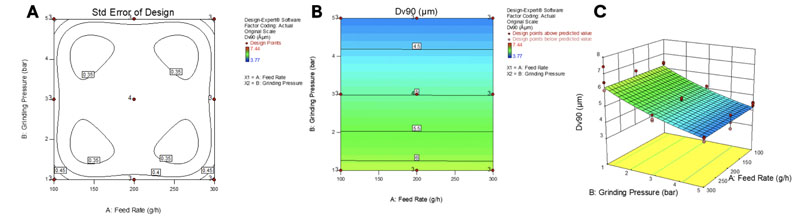
Figure 2: DoE experimental plan and standard error distribution (A), 2D contour plot (B) and 3D contour plot (C)
This model was used to target a suited-for-inhalation Dv90 of 5 µm. The feed rate was maximised and the grinding pressure adjusted. Based on the model, a feeding rate of 300 g/h and a grinding pressure of 2.7 bar were applied in a run with 20 g of material for an expected predicted Dv90 of 5.1 µm.
The generated material presented a Dv90 of 5.3µm, which was within the confidence interval of the model (95%) for 99% of the population. Thus, the obtained model proved to be predictive
Take home message
An initial jet milling feasibility study can be performed with as little as 5 g of material. Next, a DoE approach allows the process operating range to be fully characterised while testing a limited number of conditions.
This ensures that an understanding of the process is gained for a given active pharmaceutical ingredient (API). This is particularly relevant for inhalation-targeted APIs with tight specifications.
Reference
- H.K Yoon, "Effect of Nilotinib on Airway Smooth Muscle Thickening in a Murine Model of Chronic Asthma," 2012: https://erj.ersjournals.com/content/40/Suppl_56/2804.

