In this article, SiSaf’s Dr Suzanne Saffie-Siebert (CEO), Andy Gibson (Head of Manufacture) and Ashkan Dehsorkhi (Research Team Leader) explore these challenges and explain how the company’s hybrid LNP technology has been developed to overcome them.
The first LNP-formulated nucleic acid medicine was patisiran (Onpattro), an siRNA drug that silences the mutant TTR gene in hereditary transthyretin amyloidosis. Its approval in 2018 undoubtedly marked a major milestone in the field.1
However, the international rollout of the Moderna and Pfizer/BioNTech COVID-19 vaccines is what really established LNP-enabled RNA therapeutics as a new treatment modality on a worldwide scale.
There are now more than 200 ongoing clinical trials with RNA therapeutics, approximately half of which use LNPs as the delivery vehicle.2 In particular, flourishing development pipelines for mRNA cancer vaccines highlight oncology as a major future growth area.3
From a technology perspective, it took almost three decades to develop LNP formulations for the clinical delivery of RNA and several requirements are now recognised for successful clinical translation.4
These include an average particle size of ≤100 nm, high RNA encapsulation efficiency, scalable manufacturing capability and satisfactory product stability.
The last of these is highly challenging and stability problems continue to impact the main methods used to commercially produce LNPs. Moreover, these limitations hinder the rapid projected growth of the RNA therapeutics field.
The above issues inspired SiSaf to develop Bio-Courier, a delivery platform based on silicon-stabilised hybrid lipid nanoparticles (sshLNPs).

As explained below, this technology overcomes many of the limitations of current LNPs and their manufacturing processes. It could potentially revolutionise the production and distribution of LNP-formulated RNA medicines.
Basic principles of LNPs and their production
All LNPs for RNA delivery consist of four major components: an ionisable/cationic lipid, a helper lipid, a PEGylated lipid and cholesterol. Under suitable conditions, these components self-assemble into small colloidal particles that encapsulate the nucleic acid cargo.
Early production methods for nucleic acid-loaded LNPs, such as lipid thin film hydration, were important in terms of establishing proof of concept … but came with several drawbacks (such as inconsistent particle size and low encapsulation efficiency).
Solvent-injection mixing was then introduced, in which the lipid components are dissolved in an organic solvent (usually ethanol) and added to a buffered aqueous solution of the nucleic acid.5
This brought some improvements but was still unsatisfactory for pharmaceutical products owing to high batch-to-batch variation.6 The key breakthrough for scalable, commercial LNP formulations came with the development of rapid mixing methods.
Rapid mixing methods enable commercial LNP manufacturing
Crossflow injection or T-junction mixing (Figure 1a) is an adaptation of the solvent-injection approach with rapid mixing of the organic and aqueous solutions. It enables reliable production of small LNPs with the particle size controlled by the injection flow rate and pressure.7
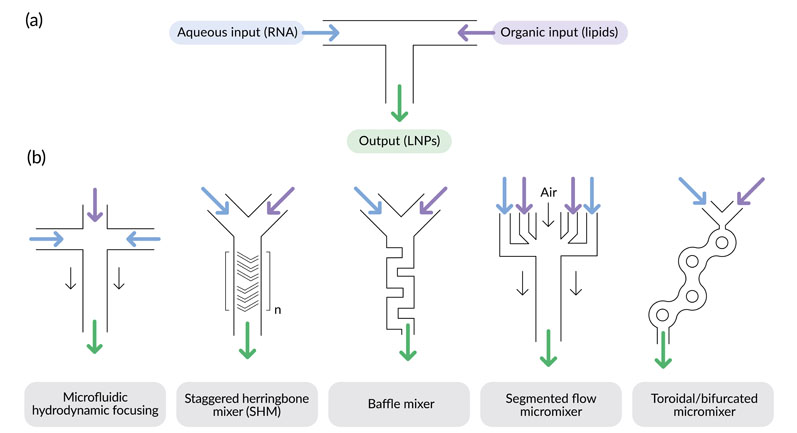
Figure 1: Rapid mixing configurations for LNP production: (a) = T-junction mixer, (b) = other mixer designs developed for use in microfluidic devices; note that for the staggered herringbone mixer, multiple mixing cycles are usually employed in series (denoted by n)
Improved control of the mixing environment ensures the production of LNPs with uniform size and properties, which is essential for consistent biodistribution and clinical performance.8
It also means that size-reduction steps are no longer required and the encapsulation efficiency is increased. Prior to fill/finish operations, the organic solvent is removed from the LNP preparation in a post-processing step, usually by tangential flow filtration (TFF).
Commercial scale-up is well-established and improved protocols continue to be developed. For example, Globe Biotech recently used the design of experiments (DoE) approach to implement a continuous manufacturing process for mRNA-loaded LNPs that meets current regulatory recommendations.9
However, even with careful optimisation, bulk production methods are prone to significant batch-to-batch variation.6 As a result, microfluidic solvent-injection mixing approaches were developed —based on the rapid mixing principle — to theoretically allow for greater process control and easier scalability.
Preclinical studies have often used mRNA-loaded LNPs produced using microfluidic devices with various mixer configurations (Figure 1b).7 Scale-up is achieved by extensive parallelisation of the microfluidic workflow and, as with T-junction mixing, the size and properties of the LNPs are directly controlled by the process parameters.7
Organic solvent removal can again be done by TFF or it can alternatively be integrated into the microfluidic workflow.

Notable examples include the invasive lipid nanoparticle production (iLiNP) device, a microfluidic system based on a baffle mixer that can precisely tune LNP size by varying the flow rate and mixer dimensions.10
The original device has recently been parallelised for large-scale production of RNA-loaded LNPs.11 Elsewhere, the staggered herringbone mixer (SHM) is a popular choice for microfluidic fabrication of siRNA- and mRNA-containing LNPs for preclinical studies.6,8
In sum, rapid mixing is the preferred manufacturing strategy for RNA-loaded LNPs, whether by bulk (T-junction mixing) or microfluidic methods.12
Although optimisation of the lipid components enabled LNPs to be deployed as RNA delivery vehicles, it was really the development of rapid mixing protocols that enabled practical clinical translation.
Nevertheless, it is important to appreciate that LNPs are not equilibrium structures and that many process parameters influence manufacturing outcomes.13 In the context of commercial production, this explains why LNP-formulated RNA therapeutics are currently manufactured in only a few dedicated facilities.14
Current landscape of LNP production
Patisiran is produced using microfluidic ethanol-injection rapid mixing.15 In contrast, the manufacturing methods for the Moderna and Pfizer/BioNTech COVID-19 vaccines have not yet been publicly disclosed.
More broadly, several companies are developing and marketing dedicated systems for clinical and commercial LNP production.7
This includes both T-junction and microfluidic platforms, as well as impinging jets mixers, which have an adapted T-junction mixer based on flash nanoprecipitation to further reduce the mixing time.16
Regardless of the underlying technology, commercial instruments for LNP production can typically handle batch sizes from 0.5 mL to 10 L.17
Thus, RNA-loaded LNPs can be manufactured using various methods from research to process scales. However, these procedures all have significant drawbacks that hinder the improved clinical translation of RNA therapeutics.
It is important to briefly explain these issues before moving on to describe how Bio-Courier technology was developed to overcome them.
Limitations of current manufacturing approaches
With RNA-loaded LNP formulations, issues can arise from either the nucleic acid cargo or the delivery vehicle itself. For LNPs, the PEGylated and ionisable lipid components create particular safety concerns.

All PEGylated lipids contain polyethylene glycols (PEGs), which are widely used biocompatible polymers that “shield” against aggregation and protein binding to improve pharmacokinetic properties.18
However, they can also be immunogenic and anti-PEG antibodies have been found in many people, meaning that current LNPs could cause severe hypersensitivity reactions in some cases.18,19
It is not easy to remove the PEGylated lipid component because it has important structural and functional roles.20
During the manufacture of LNPs, the molecular weight and molar ratio of the PEGylated lipid largely determine the resultant particle size, and this holds true for both T-junction mixing and microfluidic approaches.21,22
The ionisable lipids in LNPs can also cause problems. For example, lipid oxidation can produce chemical species that directly react with RNA to inactivate the drug cargo (with currently unknown safety implications).23
A final issue with conventional LNPs is their sensitivity to shear stress, which necessitates special modifications to the solvent removal and fill/finish steps to avoid aggregation or degradation.8,17
Although the above concerns have been minimised by extensive process and formulation development, the inherent chemical instability of RNA still presents a major manufacturing (and distribution) challenge.
Essentially, this is because all current manufacturing workflows require the RNA to be introduced during the initial formation of LNPs.8,9
The LNPs are effectively formed around the nucleic acid cargo as it cannot easily be introduced later; but, because it is chemically unstable, this restricts commercial batch sizes for mRNA-loaded products to no more than a few hundred litres.24
Even with careful process control, product quality is still compromised.
Owing to mRNA instability, the Pfizer/BioNTech COVID-19 vaccine (tozinameran) loses an estimated 30% of initial API (active pharmaceutical ingredient) integrity during the manufacturing process.25
This problem is further highlighted by the much-publicised need for end-to-end cold chain distribution for both of the approved COVID-19 mRNA vaccines (−80 °C in the case of the Pfizer/BioNTech product). Even at these low temperatures, further slow degradation is considered likely.25
Here, the molecular size of the API is important. With its much smaller siRNA active ingredient, patisiran has a shelf-life of 3 years in a refrigerator.14 Thus, RNA stability issues mostly affect mRNA-containing formulations.
These account for the majority of active clinical trials on RNA therapeutics, illustrating how more stable mRNA–LNP formulations are urgently required.2
Newer lyophilised formulations have shown much better temperature stability, but commercial scalability of these workflows has yet to be established.26 Also, they fail to address the fundamental problem of early RNA incorporation during production.
A group of researchers in Japan recently reported a method for encapsulating mRNA into LNPs by rehydrating empty, freeze-dried LNPs with mRNA solution followed by incubation at 95 °C for 5 min.27
The heating step risks RNA degradation and adds complexity to the manufacturing process, so it seems unlikely that the proposed method offers a viable way to separate LNP manufacture and RNA encapsulation.
SiSaf approached the problem by conceiving a completely different approach to uncoupling LNP manufacture from final drug product formulation.
Streamlined manufacturing with hybrid LNP technology
Bio-Courier is an emerging technology based on silicon-stabilised hybrid lipid nanoparticles sshLNPs, a novel nanomaterial for drug delivery (Figure 2).
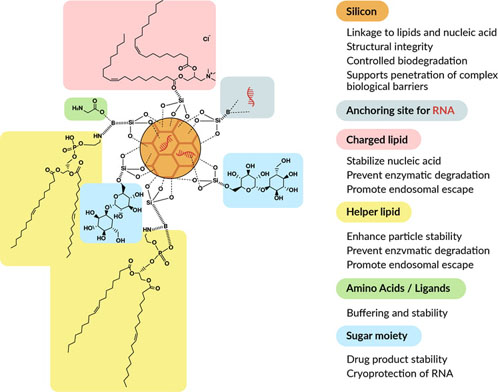
Figure 2: Schematic representation of silicon-stabilised lipid nanoparticles (Bio-Courier) for nucleic acid delivery
Lipids — typically, a combination of cationic or ionisable lipids and helper lipids — are combined with hydrolysable mesoporous silicon and stabilising agents, such as amino acids and sugar moieties.
Nucleic acid loading is done after initial production of the nanoparticles and occurs both on the surface and internally.
The technology was primarily developed for increased stability and shelf-life without the need for PEGylated lipids or cholesterol, which is achieved as a result of strong electrostatic interactions between the two lipid layers. Thus, sshLNPs differ significantly from conventional LNPs and offer a number of advantages.
First, they show improved chemical stability. The ionisable, cationic and helper lipids used in LNPs all contain ester linkages that are prone to chemical hydrolysis, limiting the stability and shelf-life of LNP formulations stored in aqueous solution.28
A recent pharmacokinetic study by Moderna also identified ester hydrolysis as a major metabolic pathway for ionisable lipids after administration … and pointed out that a better understanding of such processes is important for the wider application of LNP-enabled RNA therapeutics.29
In this context, sshLNPs could be advantageous as ester hydrolysis is reduced because of silicon–lipid binding (Figure 2).
Superior physical stability is another major feature of Bio-Courier formulations. The positively charged surface of sshLNPs (zeta potential >30 mV) prevents aggregation and enables them to remain as stable colloidal suspensions.
In long-term storage tests, they maintained their original size and zeta potential (a measure of surface structural stability) for at least 80 weeks at 4 °C and 24 weeks at room temperature (Figure 3).
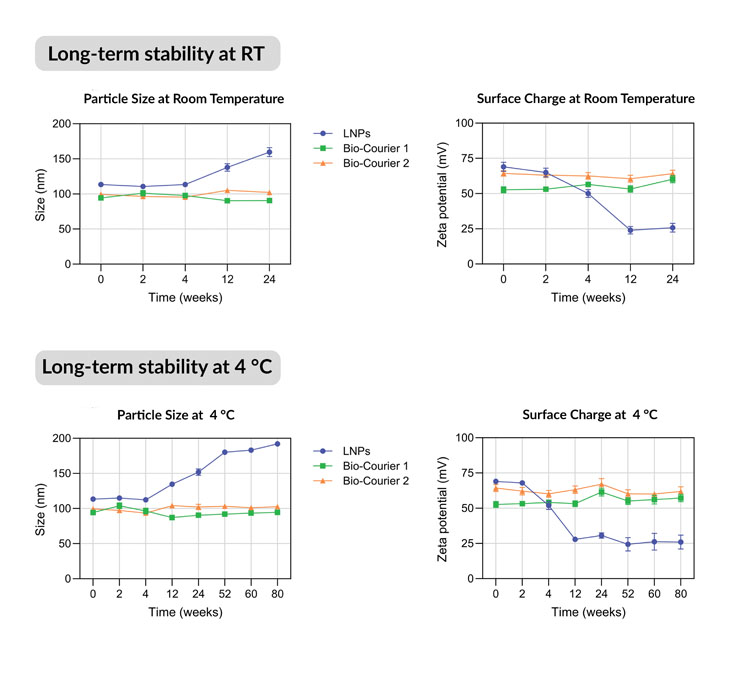
Figure 3: Long-term stability of Bio-Courier formulations compared with LNPs formulated without silicon
Non-PEGylated sshLNPs were equally stable for 80 weeks with no evidence of aggregation. In contrast, conventional LNPs gradually lost surface charge and underwent fusion/enlargement during the same timescales.
Bio-Courier formulations are also more resistant to shear stress, which is an issue for conventional LNPs during fill/finish, as noted above. For sshLNPs, the polydispersity index (PDI) was unaffected by multiple cycles of extrusion through a 30 nm membrane — unlike LNPs formulated without silicon (Figure 4).
Most importantly of all, the presence of the silicon component makes sshLNPs amenable to RNA loading after initial manufacture. This is a key differentiator from current LNP formulations.
It enables a new convergent manufacturing approach whereby “empty” nanoparticles are produced first and loaded later with any chosen therapeutic RNA, instead of the nucleic acid being incorporated during initial production.
With Bio-Courier, it is also possible to tune the nucleic acid loading and release characteristics by adjusting the chemistry of the system; for example, through the use of doped silicon (as illustrated in Figure 2) or surface functionalisation of the nanoparticles.
The loading step can be separated by considerable time and distance from original production. Small quantities of empty Bio-Courier may be used in localised centres to prepare personalised medicines for individual patients.
Equally, large quantities of lyophilised sshLNPs can be shipped anywhere in the world for reconstitution and loading in local fill/finish operations. At the point of use, RNA loading is straightforward and only needs one hour at room temperature to produce final formulations for administration.
As there is no need for cold chain distribution (as empty sshLNPs are stable at ambient temperature), this offers a more geographically accessible and sustainable alternative to current LNPs.
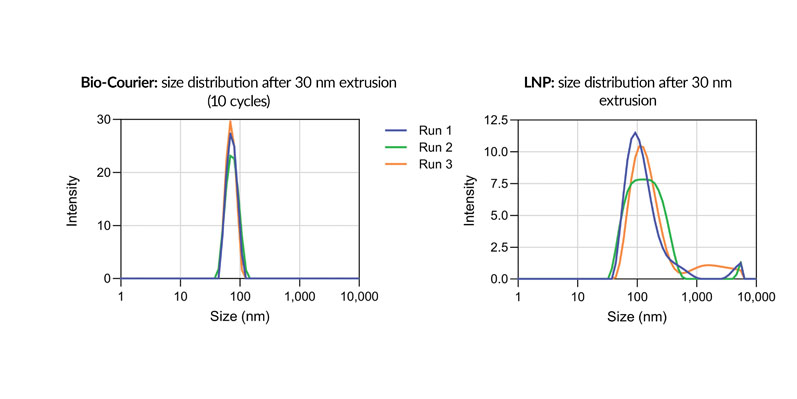
Figure 4: Size distribution of Bio-Courier formulations compared with standard LNPs after 30 nm extrusion; differently coloured plots indicate individual replicates
Cost and supply chain complexity are considerably reduced by manufacturing Bio-Courier in large batches at a few locations and shipping to local sites as required. This makes sshLNPs useful for both high-demand products and personalised medicine applications.
Late-stage API incorporation also overcomes the safety and efficacy concerns associated with gradual RNA degradation during the manufacture, distribution and storage of current formulations.
The Bio-Courier manufacturing workflow largely mirrors that of conventional LNPs, except for some adaptations that enable incorporation of the silicon component (Figure 5).
Mesoporous silicon nanoparticles are first dispersed in methanol and combined with stabilising agents. The lipid components are introduced by T-junction mixing to assemble sshLNPs, then size reduction (via membrane extrusion) and solvent removal steps provide the final product.
The optimised process works on any scale, up to thousands of litres, and delivers sshLNPs with consistent properties (size, PDI, zeta potential and lipid content).
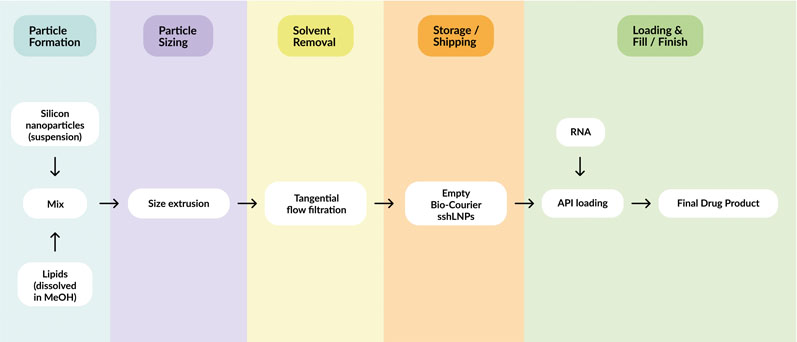
Figure 5: Schematic of the manufacturing workflow for Bio-Courier, highlighting how API loading and final drug product formulation can be separated in time and distance from initial production of the sshLNPs
Conclusions and outlook
Nucleic acid therapeutics could transform the way we protect against infectious disease and treat a range of other conditions, including many types of cancer or rare and ultrarare diseases.
Achieving this requires safe and efficient technologies that can deliver intact RNA molecules and, at the same time, allow commercially viable low-volume production. SiSaf’s Bio-Courier technology can address these challenges.
By separating initial manufacture from API loading, Bio-Courier technology could extend global accessibility to regions where end-to-end cold chain distribution is unaffordable or unfeasible and it could reduce the environmental impact associated with ultracold transport and storage.
The modular nature of Bio-Courier also opens up new possibilities in personalised RNA medicine as it permits the on-demand preparation of customised RNA-loaded nanoparticles on any scale at the point of use.
Finally, Bio-Courier is not limited to mRNA delivery, and SiSaf’s current pipeline also includes CRISPR gene editing and siRNA-based products.
We are confident that, in time, sshLNPs will establish a paradigm shift in nucleic acid therapeutics and drive the improved clinical translation of this rapidly expanding field of innovative new medicines.
References
- https://doi.org/10.1038/s41565-019-0591-y.
- https://doi.org/10.1002/btm2.10374.
- https://doi.org/10.1016/j.addr.2023.114993.
- https://doi.org/10.1038/s41565-021-00898-0.
- https://doi.org/10.1002/smtd.201700375.
- https://doi.org/10.1016/j.biomaterials.2021.120826.
- https://doi.org/10.1080/17425247.2022.2135502.
- https://insights.bio/cell-and-gene-therapy-insights/journal/article/2515/Process-analytical-insights-for-GMP-manufacturing-of-mRNA-lipid-nanoparticles.
- https://doi.org/10.1038/s41598-022-12100-z.
- https://doi.org/10.1021/acsomega.8b00341.
- https://doi.org/10.1016/j.apmt.2023.101754.
- https://doi.org/10.1038/s41578-021-00358-0.
- https://doi.org/10.1016/j.cocis.2023.101705.
- https://doi.org/10.1016/j.xphs.2022.11.001.
- https://doi.org/10.1021/acsami.0c05489.
- https://doi.org/10.1186/s12967-019-1945-9.
- https://berkshiresterilemanufacturing.com/resources/blog/beyond-lipids-the-science-of-lnp-manufacturing-and-fill-finish.
- https://doi.org/10.1021/acsnano.9b03942.
- https://doi.org/10.1016/j.jconrel.2022.09.031.
- https://doi.org/10.3390/pharmaceutics14112520.
- https://doi.org/10.1038/mtna.2012.28.
- https://doi.org/10.1039/C9NR02004G.
- https://doi.org/10.1038/s41467-021-26926-0.
- https://www.pharmasalmanac.com/articles/overcoming-bottlenecks-in-mrna-manufacturing.
- https://doi.org/10.1016/j.tibtech.2022.03.012.
- https://doi.org/10.1038/s41421-022-00517-9.
- https://doi.org/10.1021/acsnano.2c10501.
- https://patents.google.com/patent/US11173120B2/en.
- https://doi.org/10.1124/dmd.122.001194.
