Paediatric populations represent the most diverse patient groups in all of medicine. Factors such as individual preferences, physiology and dosage considerations all serve to complicate drug formulation for paediatric care, which has become increasingly important as the healthcare field recognises the huge impact that strict patient adherence can have on a treatment’s efficacy.
In addition, the off-label administration of a therapy — more common among younger and older patients — can both decrease efficacy and create the potential for medication-related toxicities.
Medication adherence is influenced by several factors: the method of treatment, healthcare accessibility, a patient’s socioeconomic status and the palatability and swallowability of a drug all play a role. For paediatric populations, adherence can be complicated by other considerations, such as a child’s ability or willingness to accommodate any bitterness or formulation texture issues.
Liquid and chewable formulations are common for paediatric medications. Although many active pharmaceutical ingredients (APIs) can be adequately masked in such formulations, numerous bitter APIs present formulation challenges that can benefit from novel taste-masking approaches.
By partnering with a contract development and manufacturing organisation (CDMO) with experience in paediatric formulation, pharmaceutical companies can increase the acceptability of their drugs among paediatric patients. Their idiosyncrasies and an increased regulatory focus on adherence both impact a pharmaceutical landscape that is also shifting toward more patient-centred at-home care.

With regulatory bodies emphasising the importance of codeveloping paediatric formulations from the beginning of a drug’s development, co-ordinated, comprehensive scale-up and manufacturing is integral to a treatment’s ultimate commercial and therapeutic success.
The factors that influence treatment adherence in children
Eating and swallowing are complex behaviours involving more than 30 muscles and nerves that perform both volitional and reflexive activities. These behaviours differ in young children compared with adults owing to changes in the pharynx that occur with maturation.
This difference can be most readily seen in infants because their teeth have not erupted, the hard palate is flatter and the larynx and hyoid bone are higher in the neck. Additionally, the epiglottis contacts the back of the soft palate such that the larynx is open to the nasopharynx but separated from the oral cavity by a soft tissue barrier.
As the neck elongates with growth, the larynx descends to a lower position in the neck and the contact between the soft palate and epiglottis is lost (while the pharynx lengthens vertically).1 These differences, taken together, can have an appreciable impact on how infants and small children swallow when compared with adults.
Consequently, developers and manufacturers should understand the impacts of physiology when formulating a drug for paediatric populations.
Texture also has an impact on the acceptability of a drug formulation, particularly for paediatric patients. To create an oral formulation that achieves consistent and broad acceptance, many drug developers have studied the commonalities that exist among children from various cultures relating to food preferences.
A common denominator for the most acceptable foods is a combination of mild flavours and smooth textures — foods such as pudding, apple sauce, custard and the like possess the broadest acceptability across countries and cultures.2 Oral formulations that mimic the texture of these foods tend to exhibit greater levels of adherence.
For older children, overall palatability is arguably more important than any other factor when deciding on a formulation approach. Once children are 5 years old, swallowing begins to more closely mirror that of a full-grown adult.3

This means that a larger contributor to potential rejection of an oral medication may come down to individual preference; although it is industry standard, for example, to assume that the maximum allowable size of an oral tablet formulation is approximately 1 g, studies show that both children and adults are capable of swallowing much larger doses.
To achieve this often requires a formulation with the texture and rheology of soft foods; children are capable of swallowing boluses in excess of 10 g, provided that they possess the characteristics of the broadly palatable foods that are most acceptable to them.4
Although formulating drugs specifically for paediatric populations is important to enhance acceptability, it also helps to discourage the off-label administration of oral medications. Crushing or otherwise compromising the composition of a drug and mixing it with food or beverages to enhance its palatability or swallowability has historically been common practice, particularly among paediatric and geriatric patients.
This sometimes harmless tampering can have appreciable effects on the potency and efficacy of a drug. The rate and extent of drug released from the formulation may be altered by the crushing process, which can result in deleterious alterations to timed release characteristics as well as increased dissolution and bioavailability, which may cause too much of a drug to be absorbed too quickly.
Either scenario can be medically serious, particularly for children who may lack both the communication skills necessary to convey their discomfort and the comprehension needed to adhere to medical protocols that cause discomfort.
Partnering for better paediatric patient outcomes
As more companies consider pursuing oral drug formulations for specialised patient populations, the cost and timelines associated with developing alternative dosage forms can result in a reluctance to invest.
Finding a manufacturing partner with the experience, expertise and facilities needed to pursue a range of formulation options can help to expedite the development process, particularly for new APIs with varying taste and solubility profiles.
Developing a target product profile for paediatric populations is a multidisciplinary exercise that involves formulation and clinical development considerations. Most clinical operations focus on the API, the dose and the side-effects of a drug.
By introducing formulation expertise to the clinical setting as early as possible, companies can achieve broader acceptance for their drug formulation while minimising the additional work required to achieve it.
Adare Pharma Solutions, a multinational CDMO providing product development through commercial manufacturing expertise, has long focused on oral dosage forms for the pharmaceutical industry. Adare’s specialised technology platforms provide taste-masking, controlled release, solubility enhancement and patient-centric dosing solutions for a wide range of pharmaceutical applications.
With a proven track record in drug delivery, Adare has developed and manufactured more than 40 products sold by customers in more than 100 countries. This global footprint has afforded Adare a unique perspective on achieving acceptable drug formulations for a variety of patient populations.
Its formulation solutions reflect its overarching focus on a patient’s quality of life: by offering a wide range of state-of-the-art taste-masking technologies, Adare can offer customers the right-fit solution for nearly any drug substance and any patient population.
Physical barriers remain one of the most efficient technologies for taste-masking and Adare offers commercially feasible physical barrier technologies, such as Microcaps and Optimμm, for taste-masking and dosage forms for paediatric populations. Both solutions boast a wide array of delivery formats, including powders, liquid suspensions, orally disintegrating and dispersible tablets.
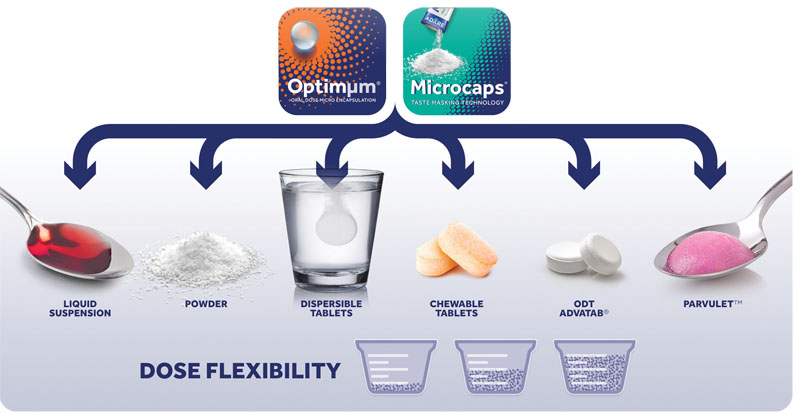
In addition, Adare’s Parvulet dosage form approximates the texture of easy-to-swallow soft foods. Parvulet initially takes the form of a granule/powder or tablet. To administer, it is placed in a spoon along with a few millilitres of water and the solid dosage form is then converted into a food-like product with the consistency of apple sauce.
Converting the solid dosage form into a bolus-like texture takes less than a minute. It does not require any mixing or caregiver action that could increase the risk of human error or impact dose integrity. The converted product also has the advantage of preventing any spills as the swollen mass does not fall out of the spoon (even when turned upside down).
APIs that have been taste-masked via Microcaps or Optimμm can be incorporated into the Parvulet composition to leverage the dosage form’s ease of administration and improve patient compliance.
Each technology offers a range of benefits: Adare’s Microcaps technology, which involves the uniform coating of a solid particle or liquid droplet with a rigid, semipermeable polymer, allows developers to combine incompatible APIs, customise a drug’s release profile and effectively taste-mask a range of bitter molecules.
Optimμm technology, which can generate uniform microspheres and microcapsules, offers tremendous uniformity, dose and format flexibility, tailored release kinetics and taste-masking. The particle size and particle size distribution for Microcaps and Optimμm allow both to avoid the “sandy” effect sometimes found in powders and optimise their dissolution profile.
Microcaps and Optimμm represent just two of Adare’s solutions to increase palatability. When these technologies are combined with the Parvulet composition, swallowability is significantly increased, which helps to overcome the challenge of dosing patients with swallowing problems.
Adare’s portfolio represents one of the most comprehensive in the world regarding taste-masking and enhanced product acceptability. Its technologies, coupled with its long-standing focus on patient compliance, have positioned Adare as a leader in formulation for specific populations.
Its expertise extends to paediatric formulations, which can carry complexities that are challenging for companies new to pursuing patient-centred development. By partnering with a CDMO such as Adare, companies can help to ensure that their drug’s formulation is the best fit for its target patient population, meets increasingly stringent regulatory standards and, ultimately, improves therapy outcomes through better patient adherence.
References
- K. Matsuo and J. B. Palmer, Phys. Med. Rehabil. Clin. N. Am. 19(4), 691–707 (2008).
- J. Guinard and R. Mazzucchelli, Trends in Food Science & Technology 7(7), 213–219 (1996).
- R. Shaker, et al., Principles of Deglutition: A Multidisciplinary Text for Swallowing and its Disorders (Springer Science & Business Media, Berlin, Germany, 2012).
- A.M. Wintergerst, et al., CRANIO 34(4), 257–263 (2016).
