Gene therapy shows great promise as a tactic for the treatment of many diseases, including cancer. For example, approaches that use targeted systems to deliver oligonucleotides offer the potential to overcome the drawbacks and limitations seen with conventional small molecule drugs.
In fact, it’s been said that the recent approvals of the first RNA-based drugs mark the beginning of a therapeutic revolution, the likes of which have not been seen since the advent of recombinant protein technology almost 50 years ago.1
To add some context, if we consider the global market for siRNA therapeutics, industry researchers suggest that, by 2027, its value will reach $724.3 million, representing a CAGR of 7.62% during the 2020–2027 period.2
RNA therapies, from antisense nucleotides to siRNAs, either intend to alter cell protein expression by introducing exogenous genetic material — which then disrupts or reduces the transcription of a target gene — or disrupt the translation of faulty messenger RNA (mRNA).
In addition, mRNA therapeutics are designed to replace defective proteins or present antigens for vaccination. However, to achieve these effects, a significant central development and formulation challenge must be overcome: the need to protect sensitive nucleic acids from enzymatic degradation in the body and deliver them to a target site while minimising off-target effects.
Challenges of chemotherapy and the promise of new therapies
Although conventional chemotherapy drugs are still the mainstays of oncology treatment, their mechanism of action — attacking the mitotic cycle — limits their potential in two ways.

The indiscriminate destruction of both malignant and normal cells restricts the dose that a patient’s body can withstand (owing to the toxicity to normal cells). This also confers the risk of serious short- and long-term side-effects, such as life-threatening infection and cardiac damage.
At the same time, traditional chemotherapy drugs typically lack the capacity to wipe out 100% of malignant cells, something that can ultimately lead to the development of drug resistance. The resulting ineffectiveness of the drug treatment remains a major problem in oncology, with reports suggesting that it accounts for up to 90% of cancer-related deaths.3
A further challenge with many existing chemotherapeutic drugs is that they are poorly water-soluble and must be formulated using high concentrations of surfactants and co-solvents, which frequently lead to adverse side-effects.4
A lack of good aqueous solubility has been cited as a key obstacle in the development and clinical use of anticancer compounds ranging from taxanes and platinum-based agents to drugs that have been approved for other indications and could potentially be repurposed for cancer treatment.5
In addition, as conventional chemotherapeutic agents are typically delivered systemically, only a small amount of the drug actually reaches cancer cells. Furthermore, the dissemination of drugs into tumour tissue varies greatly and may not correlate with measures such as plasma concentration.6
Moreover, poor blood supply in solid tumours can prevent a drug from penetrating the core of the tumour. All these factors can impair treatment efficacy, ultimately resulting in low survival rates.
Newer targeted therapies have overcome some of these obstacles in the treatment of certain cancers. For example, antibody-drug conjugates, which are considered to be the “biological missiles” of cancer therapy, are designed to deliver a toxic payload of a small molecule drug selectively to cells expressing a target antigen that is preferentially expressed in specific tumours.7
Now one of the fastest-growing drug classes in oncology, these treatments can widen the therapeutic window and reduce the chance of the off-target effects common to systemically delivered oncology drugs.
Antibody-drug conjugate (ADC) agents have improved outcomes for subsets of patients with certain cancers; for example, the success of two anti-HER-2 ADCs has transformed the treatment of HER2-positive breast cancer, a subtype with a historically poor prognosis.8
However, as the antigens in present-generation ADCs are often not target-specific, they too can cause severe side-effects, such as thrombocytopenia and liver damage.9 ADCs also suffer from issues associated with intratumour heterogeneity.10

The sheer complexity of the process of payload delivery (all four components — antigen, antibody, linker and cytotoxic drug — must be optimised) makes it difficult to ensure that each step occurs consistently.11 For example, it has been estimated that even if an ADC works at 50% efficiency, only 1–2% of the cytotoxic payload will reach the tumour cells.12
Gene therapy holds the promise to treat cancer with exacting precision by transferring therapeutic nucleic acids to tumour cells or correcting defective genes; it has grown to include a variety of approaches, including tumour suppressor gene therapy, gene silencing therapy and suicide gene therapy.
Olignonucleotide therapies are particularly well-suited for precision and personalised medicine as they can target specifically distinct transcript isoforms of key enzymes.
Currently a predominantly viral vector-based medicine, gene therapy has yielded treatments for cancers such as melanoma, soft tissue sarcoma and pancreatic cancer.
Yet, owing to their very nature, these vectors can cause immune system reactions that result in side-effects such as fever, severe chills, nausea, vomiting and headache.13
In addition, viral vectors carry a risk of recombination with endogenous viruses; methods to produce them are currently costly and have associated safety risks.
A useful review, published in 2021, summarises the history and current landscape of delivery systems for nucleic acid therapeutics — including four platform technologies that have enabled the clinical translation of nucleic acid therapeutics: antisense oligonucleotides, ligand-modified small interfering RNA conjugates, lipid nanoparticles (LPNs) and adeno-associated virus vectors.14
Solving the delivery challenge
Many years of experience and data mean that, in numerous research situations today, it is LPN technology that is selected for investigation with regards to delivery formulation.
Originally developed as a delivery strategy for poorly soluble small molecules, in which a drug was “encapsulated” within the LPN, strategies have emerged that allowed successful formulations of DNA-based drugs to be achieved. A 2019 review paper illustrates the range of LPN structures and approaches that have been employed.15
Importantly, the authors describe clearly how, even when a satisfactory formulation is achieved, this approach does not allow the precise control of possible biological response following administration — with issues of toxicity and immunogenicity being a serious limitation.
They also highlight issues of cost, reliability and scalability of manufacture as potential barriers to adoption. Against this background, silica nanoparticles can offer development and formulation scientists an alternate delivery vehicle with distinct advantages compared with systems based on LPNs.
In particular, one new class of silica nanoparticles, specifically engineered for use as a drug delivery platform, exhibits favourable performance in a series of proof of concept and preclinical studies.
Known as Nuvec, the particle has a novel surface structure that can be functionalised with PEI (polyethyleneimine) to strongly bind and protect oligonucleotides to help delivery to the cell membrane.
Crucially, it has been shown that this novel topography protects the plasmid DNA from nuclease degeneration. Upon reaching its target, the particle has strong cellular uptake based on charge attraction: it efficiently attaches itself to the cell membranes and enters the cell nucleus. Figure 1 illustrates the process of cellular uptake, endosome escape and nucleic acid release, and protein antigen expression.
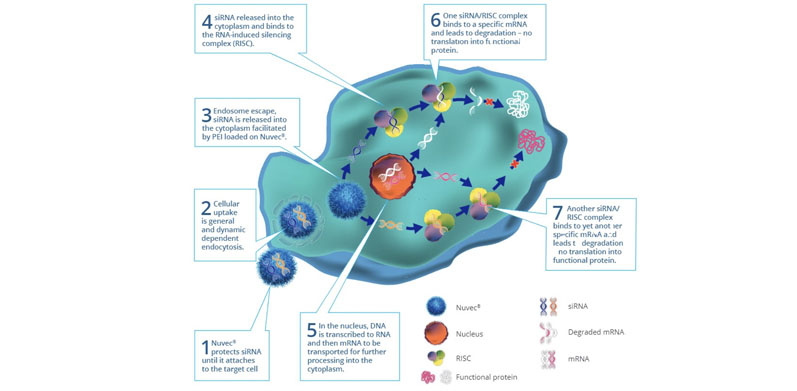
Figure 1: Schematic to illustrate the Nuvec mode of action
A significant benefit, confirmed in preclinical work, is a loading capacity higher than that of alternative silica nanoparticle shapes. It is also versatile in that it can be loaded with multiple plasmids, both small and large.
Moreover, in animal model studies, when given a single IV injection and the followed with time, no significant changes in silica levels were detected in the plasma, liver, spleen and brain versus controls.
In the context of siRNA delivery, two siRNA molecules can be loaded. When delivered to the target cell, both can then act to modulate specific intracellular pathways. This allows for the targeting of specific tumours and the inhibition of identified signalling pathways.
A further interesting property is Nuvec’s thermostability. It can be freeze-dried, stored at room temperature or at –4 °C, and reconstituted up to 30 days later without degradation and loss of function of the nucleic acid payload.
Conclusion
To date, companies developing DNA/RNA-based oncology drugs or vaccines have had a limited choice of delivery systems to work with — LPNs, viral vectors or electroporation being the established choices.
Although these can produce good results, there are significant challenges to overcome, such as undesirable side-effects (accumulation in the liver), the infection of healthy cells and the stimulus of unwanted immune cell activity.
In addition, the process and cost of manufacture of LPNs limits their application. With Nuvec, we aim to offer R&D and formulation scientists a viable and powerful alternative that addresses these issues and look forward to a future wherein Nuvec has enabled and accelerated the introduction of novel siRNA (and other nucleic acid based) therapeutics.
References
- www.frontiersin.org/articles/10.3389/fbioe.2021.628137/full.
- BrandEssence Market Research, Small Interfering RNA (siRNA) Therapeutics Market by Product Type, Application and Industry Size, Share, Trends Analysis (2021–2027) | Report Analysis 2021-2027 (brandessenceresearch.com).
- https://cdrjournal.com/article/view/3039.
- www.ncbi.nlm.nih.gov/pmc/articles/PMC4113620/.
- www.ncbi.nlm.nih.gov/pmc/articles/PMC4113620/.
- https://ascpt.onlinelibrary.wiley.com/doi/10.1002/cpt.1211.
- https://pubmed.ncbi.nlm.nih.gov/34641391/.
- https://breast-cancer-research.biomedcentral.com/articles/10.1186/s13058-021-01459-y.
- https://link.springer.com/article/10.1007/s40264-018-0775-7.
- https://pubmed.ncbi.nlm.nih.gov/34112795/.
- https://dailynews.ascopubs.org/do/10.1200/ADN.20.200278/full/.
- https://pubs.rsc.org/en/content/chapterhtml/2019/bk9781788010771-00001?isbn=978-1-78801-077-1.
- www.oncolink.org/cancer-treatment/gene-immunotherapy/what-is-gene-therapy#:~:text=After%20initially%20receiving%20a%20type,48%20hours%20of%20the%20infusion.
- www.nature.com/articles/s41565-021-00898-0.
- www.ncbi.nlm.nih.gov/pmc/articles/PMC6723796/.




