With any healthcare treatment, patient compliance is key. However, according to NICE (the National Institute for Health and Care Excellence), between a third and a half of all medicines prescribed for long-term conditions are not taken as recommended.1
Although the NICE definition of medicines includes oral tablets, syrups, ointments, eyedrops and suppositories, this article will focus on how compliance with oral tablets might be improved, particularly among patient groups who struggle to take regular pills.
There are many patients who have difficulty swallowing (dysphagia), whereas others may have format, taste and texture preferences. To promote compliance among these patients — as well as others — formulators have developed new tablet formulations including minitablets, orodispersible tablets (ODTs) and orodispersible minitablets (ODMTs).
Minitablets
There is no universal definition for minitablets, but they are generally accepted to be compressed tablets with a diameter of 2–3 mm (Figure 1). Studies have demonstrated that they are a valuable alternative to syrup for young children and may in fact be more acceptable than liquid formulations.2 They could also be a suitable administration form for other patient groups with dysphagia and the elderly.
Figure 1: Minitablets are generally accepted to be compressed tablets with a diameter of 2–3 mm
Minitablets can be manufactured using regular rotary tablet presses and the use of appropriate multitip punches (Figure 2). Owing to their small size, their production can present challenges with respect to blend uniformity, flow properties and mass uniformity. The choice of filler-binder is therefore important: it needs to have powder and compression characteristics that promote both high content uniformity and relatively high tablet hardness at low compression forces.

Figure 2: Minitablets are manufactured on regular rotary tablet presses using multitip punches (centre)
Orodispersible tablets and orodispersible minitablets
Orodispersible tablets (ODTs) are tablets that disintegrate or dissolve rapidly in the mouth without the use of water or chewing. They are particularly advantageous for paediatric and geriatric populations. When delivered in minitablet form, the disintegration time can be reduced significantly.
The development of orodispersible minitablets (ODMTs) for paediatric use has gained importance in recent years; one study investigated the feasibility of isomalt, which is known for its pleasant sensory properties, as a filler-binder.3
In this study, the active pharmaceutical ingredient (API) was enalapril maleate, an angiotensin converting enzyme (ACE) inhibitor that’s used to treat various conditions, including heart failure, high blood pressure and hypertensive emergency. It is listed as an essential medicine, emphasising the need for a suitable dosage therapy for children.4
Isomalt is a polyol derived from sucrose and comprises two mutually diastereomeric disaccharide alcohols: 6-O-α-D-glucopyranoside-D-sorbitol (GPS) and 1-O-α-D-glucopyranoside-D-mannitol-dihydrate (GPM). Agglomerated isomalt is widely used as a filler-binder in direct compression tableting owing to its good compressibility, flowability and workability.
The ratio of GPS to GPM can influence isomalt’s water solubility. Isomalt with a ratio of 1:1 GPS to GPM has an aqueous solubility of 25 g in 100 g at 20 °C, whereas isomalt with a ratio of 3:1 GPS to GPM has an aqueous solubility of 42 g.
Table I shows the typical powder characteristics of each. Figure 3 shows the porous structure and spherical particle shape of each type – characteristics that promote good blending and high content uniformity in powder mixtures. To develop the ODMTs for this study, the higher solubility agglomerate form (3:1 GPS:GPM) was used.
Table I: Typical agglomerated isomalt characteristics
|
|
1:1 GPS:GPM |
3:1 GPS:GPM |
|
Solubility in water at 20 °C (g/100 g) |
25 |
42 |
|
Bulk density (g/L) |
400 |
400 |
|
Tapped density (g/L) |
480 |
480 |
|
Angle of repose (°) |
38 |
37 |
High content uniformity and low mass variation while using multitip punch tooling are critical quality attributes for low dosage minitablets. The ability to achieve content uniformity by direct compression is linked to the physical properties of the API and the excipients, the amounts present, the resulting flowability, the mixing and the tableting process.
To produce ODMTs containing enalapril maleate with a dosage strength of 1 mg for this study, enalapril maleate (16 %), 3:1 GPS:GPM agglomerated isomalt (79 %) and cross-linked polyvinylpyrrolidone (4 %) as a superdisintegrant were mixed together in a Turbula mixer for 15 minutes.
Magnesium stearate (1 %) as a lubricant was then added and the formulation was mixed for an additional 2 minutes. This blend was then compressed on a rotary tablet press into 6.25 mg round biconvex tablets using 2 mm 19-tip punches and a compression force of 5–6 kN (71–85 MPa).

Figure 3: Scanning electron micrograph images of agglomerated isomalt: Left = 1:1 GPS:GPM; Right = 3:1 GPS:GPM (Philips XL-30 FEG)
Study results
The tablets were tested to determine their disintegration time, dissolution kinetics, mass variation, content uniformity, acceptance value and some stability attributes. The disintegration time was measured using a method developed by Kleinebudde.5
One ODMT was placed into a Plexiglas cylinder, which was then locked with a 710 µm mesh sieve at the top and bottom. The locked cylinder was placed into conventional disintegration apparatus and weighted with a metal cover. The disintegration time of six ODMTs was measured at 37 ± 2 °C in demineralised water.
The dissolution tests were performed in a basket apparatus according to the USP 39-NF 34 monograph. API release was measured in a phosphate buffer (pH 6.8) stirred at 50 rpm. The wavelength was set at 208 nm.
Mass variation was performed inline with Ph.Eur. 2.9.5. Twenty minitablets were randomly chosen and weighed on an analytical balance. Content uniformity was determined using high performance liquid chromatography with UV-VIS coupling developed by Thabet and Breitkreutz.6
To determine the acceptance value of enalapril maleate according to Ph.Eur. 2.9.40, ten minitablets were dissolved in the mobile phase of a 50/50 acetonitrile/phosphate buffer (pH 2.2) and filtered through a 0.45 µm polypropylene membrane filter before injecting 20 µL of this solution.
The investigations were done in triplicate on a Nucleosil RP-18 column (240 x 4 mm, 5 µm pore size) at a temperature of 30 °C and a flow rate of 0.7 mL/min. The wavelength to measure the absorption was set at 220 nm.
To determine the stability of the ODMTs, tensile strength, API release and disintegration time were measured. Each batch was stored in a polyethylene bag under ambient conditions, with unprotected samples also tested under accelerated stability conditions of 40 °C/75% relative humidity following WHO stability guidelines.7
The batches stored under ambient conditions were analysed after 5, 6 and 7 months, whereas the samples stored in accelerated conditions were analysed after 1, 2 and 3 months. The tablet properties are shown in Table II. The dissolution measurements are shown in Figure 4.
|
Property |
Method |
Initial |
After 6 months |
|
Hardness (N) |
Ph.Eur. 2.9.8 |
4 ± 1 |
4 ± 1 |
|
Disintegration time (s) |
Modified method Ph.Eur. 2.9.1 |
9 ± 2 |
7 ± 3 |
|
Acceptance value (AV) |
Ph.Eur. 2.9.40 |
15 |
|
|
Mass |
Ph.Eur. 2.9.5 |
Conforms |
|
Table II: Tablet properties
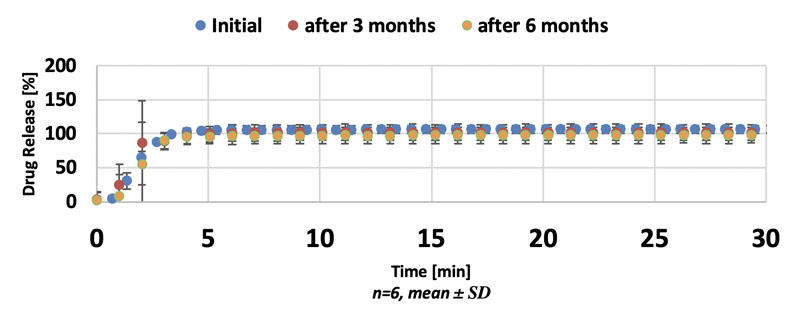
Figure 4: Dissolution of enalapril maleate ODMTs
The results show that it’s possible to obtain sufficiently hard enalapril maleate ODMTs at a low compression force. The content uniformity of the ODMTs showed an acceptance value (AV) of ≤15, which is within the specification requirements. Their variation complied with the Ph.Eur.
During the stability test period, the disintegration time of the ODMTs changed slightly under both storage conditions. However, initial and post-stability measurements showed that they fulfilled both the Ph.Eur. disintegration limit of 180 s and the US FDA disintegration limit of 30 s.
This study demonstrates that low-dose enalapril maleate orodispersible minitablets (ODMTs) can be successfully produced by direct compression using agglomerated isomalt as a filler-binder. Therefore, the agglomerated isomalt grade galenIQ 721 can be recommended as a filler-binder for the convenient manufacture of orodispersible minitablets.
References
- www.nice.org.uk/guidance/cg76/chapter/introduction.
- V. Klingmann, et al., “Favorable Acceptance of Minitablets Compared with Syrup: A Randomized Controlled Trial in Infants and Preschool Children,” The Journal of Pediatrics 163, 1728–1732 (2013).
- A. Lura, et al., “New Orodispersible Minitablets for Paediatric use – A Comparison of Isomalt with a Mannitol Based Coprocessed Excipient,” International Journal of Pharmaceutics 572, 118804 (2019).
- www.who.int/selection_medicines/committees/expert/20/EMLc_2015_FINAL_amended_JUN2015.pdf?ua=1.
- P. Kleinebudde, “Pharmazeutische Pellets durch Extrudieren/Sphäronisieren: Herstellung, Eigenschaften, Modifizierung,” Habilitation Thesis (1997).
- J.B. Yasmin Thabet, “Orodispersible Films: Product Transfer from Lab-Scale to Continuous Manufacturing,”International journal of pharmaceutics 535, 285–292 (2018).
- www.ema.europa.eu/en/ich-q1a-r2-stability-testing-new-drug-substances-drug-products.
For more information
- Oliver Luhn, Head of Pharmaceutical Technology
- Maj-Britt Cepok, Head of Business Development, Pharma
- Michael Black, Head of Sales, Pharma




