Advances in compaction science have been instrumental in improving tablet product development, scale-up, and manufacturing. Today, a variety of tools can help formulators, product developers, and other industry professionals better understand the manufacturing science and engineering behind this ubiquitous solid dosage form. These tools enable us to study tablet performance at the development stage using small quantities of material.
This whitepaper describes how to conduct common compression studies, including how to develop profiles for tabletability, compactibility, and compressibility. Those terms—which are commonly misused—have specific meanings and other different ways to characterise the material under compression.
Many factors influence successful tablet manufacture, including a properly trained staff, a table press engineered for your product’s specifications, high-quality and precisely machined compression tooling, and powder with favourable characteristics. In fact, variability in powder performance is common issue across the pharmaceutical and dietary supplement industries. A number of techniques can help you understand and resolve problems with powders. First, however, make sure that a poor-quality tablet press or substandard compression toots are not part of the equations. You need good, reliable equipment to remediate table manufacturing issues, which typically arise unexpectedly.
While understanding tablet compression is critical, the processes that precede tableting are also very important. You much understand how particle and powder characteristics affect a tablet’s quality attributes.
Particle characteristics include shape, size and size distribution, and variability in those properties can cause issues with weight, content uniformity, segregation, compression, and dissolution. The presence of a few overly large particles, for example, can lead to lower tablet weights because tablet dies are filled volumetrically. The large particles also could prolong dissolution because they would decrease the tablet’s total surface area. The presence of overly small particles would have the inverse effect.
The powder’s characteristics—including its flowability and the bulk, tapped, and true density—will also have an impact on the tableting process. True or absolute density—the density once all voids are removed—can be measured using a helium pycnometer. This is a useful value because it represents the tablet’s density if the compression events were to expel all the air between the particles, including from inter-particulates and intra-particulate voids.
Once you’ve characterised the particles and powder, decide whether direct compression—the simplest approach—is acceptable. If not, you’ll need to engineer the powder, typically by dry or wet granulation.
You much also understand how the powder behaves under different loading conditions. It’s the only path to the success in producing a robust tablet that you can scale to high-speed manufacturing. Ideally, you will learn how the material deforms to help you develop the formulation.
Tablet compression process
The studies described here were performed on a single-station tablet press. But whether your compression machine uses a single punch and a hand-filled die or is a rotary press with a gravity or force-feeding system, the process is similar: The material undergoes compressive forces in a confined space, causing a reduction in volume. At first, the low applied pressure causes particles to rearrange and pack more closely, and porosity thus decreases. As pressure increases, the dimensions of the particles change. They can either fracture into smaller discrete particles (fragmentation) or they undergo a temporary (elastic) or permanent (plastic) deformation into new shapes. At higher pressure, the particles that fracture could further rearrange and undergo additional deformation.
Tablets made from materials that undergo fragmentation are typically less sensitive to weakening when powdered lubricants (i.e., magnesium stearate) are added. Lubricants commonly inhibit bonding between particles, but when the particles fragment, new and clean surfaces are exposed, strengthening the bond. Particles that fragment are also less sensitive to the speed of the compression event. Materials that undergo plastic deformation are known for creating high-strength compacts at relatively low applied pressures, which stems from the greater number of inter-particulate contact points during the deformation process. That strength, however, may decrease when lubricants are added and when the rate of compression increases. Elastic deformation refers to a particle’s partial or full recovery to its original shape after compression loading ceases. Time-dependent elastic recovery is called viscoelasticity, and the factors that influence compression time are press speed, turret velocity, compression roller size, and punch head dimensions.
Tablet compression studies
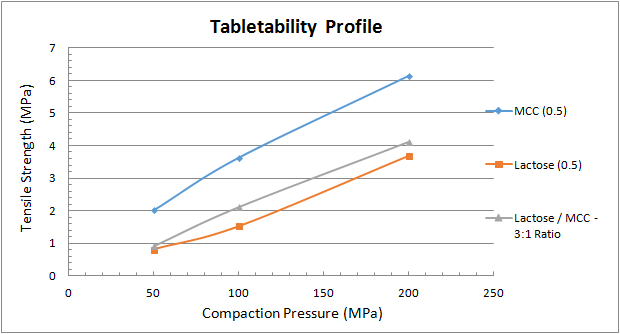
To conduct a compression study, select the tablet’s target weight and don’t change it. Next, collect samples of tablets made at different compaction forces (low to high) and measure their thickness, weight, breaking force (sometimes called hardness), and friability, as well as their disintegration and dissolution characteristics. Then plot each attribute or characteristic as a function of the compaction force. Figure 1 shows a tabletability profile, which plots tensile strength as a function of the applied compaction pressure.
The compaction pressure, which can be calculated from the applied force and cross-sectional area of the punch face (Equation 1), allows you to compare the loading of different sizes of tablets.
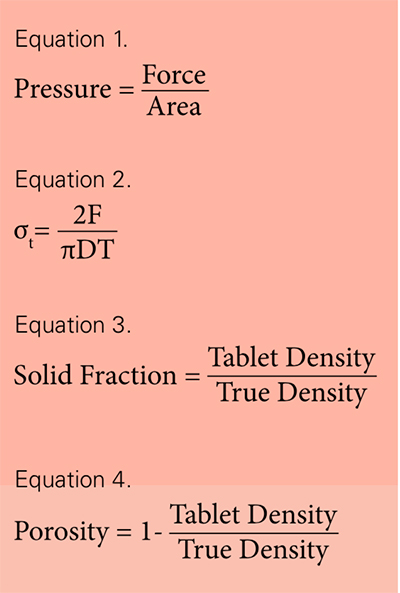
Tensile strength, which can be calculated from the breaking force and tablet dimensions, allows you to compare the mechanical strength of different sizes of tables. The tablets for the experiments summarised here were made using round, flat-faced punches and the values were calculated using the Fell-Newton equation [2], where F is the breaking force, D is the diameter of the tablet, and T is the tablet thickness (Equation 2).
Compaction pressures of 100 to 200 megapascals (MPa) are typical for pharmaceutical tablets because they may contain less than 10 percent active ingredient and a high percentage of binders, while pressures greater than 200 MPa are common for dietary supplement tablets to the very high percentage of active ingredients. Many factors will determine the target tablet strength, although 1 to 2 MPa of tensile strength is typically required for the tablet to remain intact through post-compaction, such as coating, transport/handling, and packing. From the example in Figure 1, it’s clear that from a mechanical strength evaluation microcrystalline cellulose (MCC) [3] with 0.5 percent magnesium stearate [4] provides the strongest compact. This is mainly due to the plastic deformation characteristics of MCC. The lactose monohydrate [5] with 0.5 percent magnesium stearate also provides a robust tablet at reasonable compaction pressures. But by blending the two excipients it’s possible to add multiple functions to the formulation’s performance, including plastic and brittle deformation characteristics. A 3-to-1 ratio of lactose-to-MCC provides a slightly stronger tablet than one made from pure lactose.
With the evaluation of the mechanical strengths of the tablets at different compaction pressures complete, we can turn to evaluating the solid fraction. It indicates how much of the tablet’s total volume is solid material. The solid fraction of a tablet corresponds to the ratio of the tablet’s apparent density and absolute density, as measured by a helium pychometer [6]. The tablet’s density can be calculated from its weigh and volume. The converse of density is porosity—the total volume occupied by voids of air—and it is calculated by subtracting the solid faction from 1. (see Equations 3 and 4).
Figure 2 depicts a compactibility profile, which is the tablet’s tensile strength as a function of the solid fraction. The MCC behaviour is consistent with the tabletability profile we calculated above and yields the strongest compact, in this case at the lowest solid fraction. The 3-to-1 blend of lactose-to-MCC provides a stronger compact than lactose does at a given solid fraction.
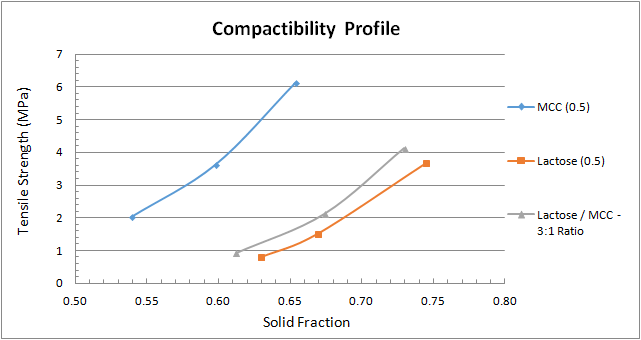
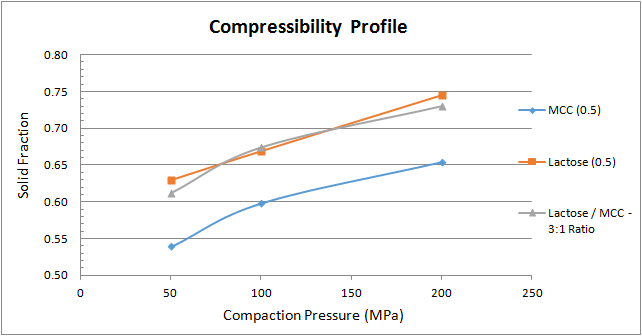
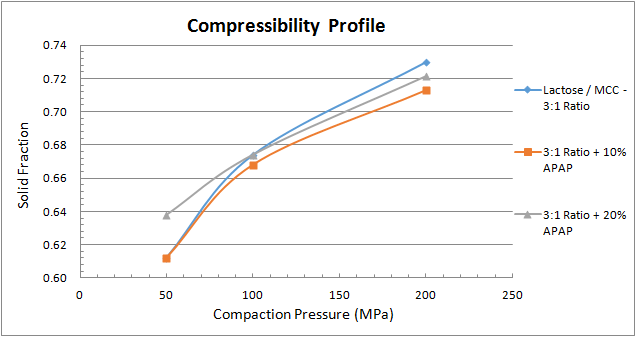
Figure 3 depicts the compressibility profile, which assesses how readily the material undergoes a change in volume when compressed. As the applied compaction pressure increases, so does the material’s solid fraction. At a specific compaction pressure, the MCC yields the lowest solid fraction, whereas the lactose and the 3-to-1 lactose-to-MCC blend provides very similar solid fraction results.
Now, that we know the compaction characteristics of the excipients, let’s add an API to the mix. Figure 4 depicts the tabletability profile of the:
- 3-to-1 lactose-to-MCC blend
- The 3-to-1 blend with the addition of 10 percent acetaminophen (APAP)
- The 3-to-1 blend with the addition of 20 percent APAP
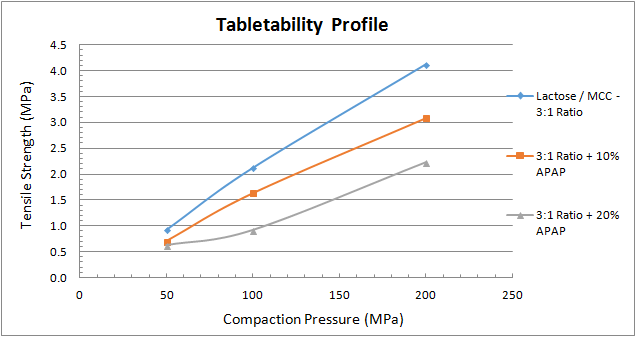
If a tensile strength of 2 MPa is the target value, the 3-to-1 blend without APAP requires onlu 90 MPa of compaction pressure, while the addition of 10 percent APAP blend requires 120 MPa, and the 20 percent APAP blend requires 180 MPa. Clearly, the APAP diminishes the tablet’s strength, so making a tablet containing APAP requires a higher compaction pressure to achieve the target strength.
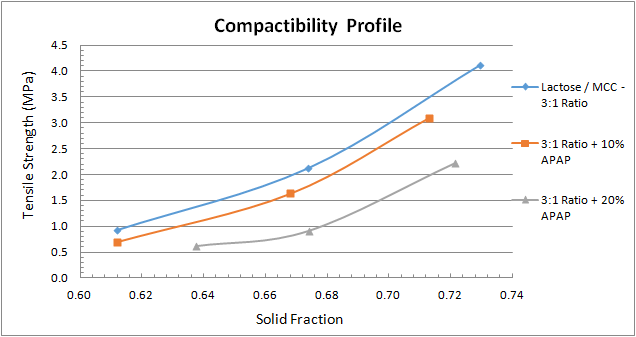
Figure 5 shows the compactibility profile of the 3-to-1 lactose-to-MCC blend and how it changes with the additions of APAP. At a given solid fraction, the addition of APAP diminishes tablet strength. Finally, the compressibility profile in Figure 6 shoes similar results for all three combinations.
Conclusion
Advances in compression science continue to help formulators succeed in delivering a branded or generic medicine. And evaluating tabletability, compactibility, and compressibility reveals the fundamental mechanical behaviour of your product in a normalised format. This approach can form the basis for developing robust products using only small amounts of material. To learn more about these evaluations and other aspects of tableting science, contact the Natoli Institute for information about its contract development services and hands-on training, which includes formulation development and scaling up to a manufacturing process.
