These small molecules and biologics form the basis of various innovative drug products including antibody-drug conjugates (ADC) and emerging drug substances used to treat autoimmune diseases. In fact, highly potent compounds now comprise over 30% of the pharmaceutical industry’s drug pipeline(1).
Despite the enormous opportunities presented by HPAPI development and manufacturing, extreme care must be taken during the process development phase. Potent APIs consist of highly toxic payloads which can be extremely hazardous at low concentrations. The Occupational Exposure Limit (OEL) for ADCs, for example, is below 0.1 μg/m3 (2). So, how do contract manufacturers and pharmaceutical companies establish appropriate engineering controls to ensure safe handling and eliminate cross-contamination of HPAPIs?
Agitated nutsche filter dryer (ANFD) systems with suitable isolator designs provide the appropriate level of containment needed for safe and effective HPAPI development.
Understanding ANFD Containment
ANFD systems are used throughout the HPAPI pipeline, both in intermediate and final product manufacturing. In either case, products with highly toxic payloads are discharged from a plug and need to be contained in an effective way, either before being looped back to an earlier phase of development or being filled into packaging containers. Conventional ANFD systems comprised a vessel with a piston valve that opened to discharge products, but such designs often require manual intervention due to residual product heel. Naturally, this wouldn’t satisfy current regulatory requirements for HPAPI manufacturing, nor would manufacturers wish to leave this heel behind as it could constitute hundreds or thousands worth of market value product. The plug needs isolating so that operatives are not exposed–but not all containment systems have been created in the same way.
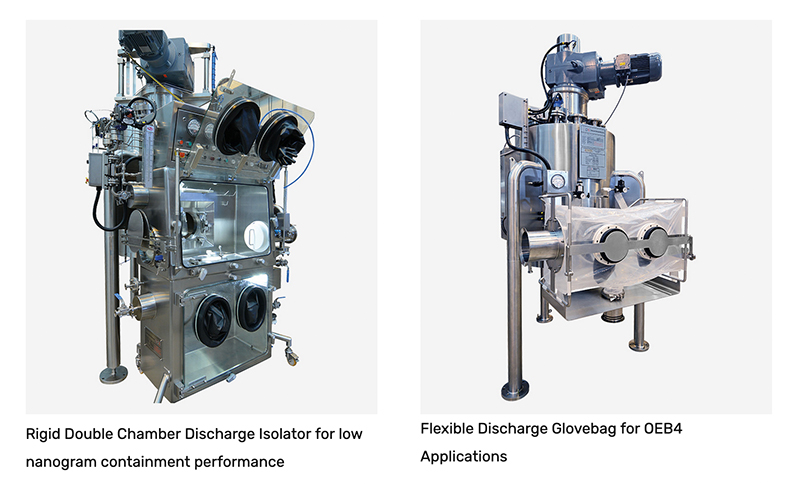
One approach is to isolate the process using a flexible container, however, this would be unsuitable for any compounds classified above Occupational Exposure Band (OEB) 4. Greater containment requires not only a rigid isolator but also air management capabilities. A single chamber glove box with a negative pressure environment and added safety features may be suitable for HPAPI containment. Yet there are additional considerations to bear in mind beyond physical isolation and air management. A single chamber may have a packaging arrangement such as a continuous liner, rapid transfer port, or split valve technology. Another solution is a double chamber arrangement wherein the product is discharged into a primary chamber where it is packaged, before moving into a secondary chamber. This is a high-end but extremely effective solution for high containment performance. Lastly, the newest and most intensive approach to HPAPI manufacturing is total process isolation.
Selecting the Right HPAPI Development Solution
Choosing the right type of containment is critical, but it is not a simple selection process. Limiting initial sources of contamination is paramount, meaning containment and air management around the discharge plug is at a minimum. However, equipment manufacturers must anticipate critical process parameters to ensure the container operates effectively. Products that are discharged as a powder, for instance, must be discharged gently to control entry into the glovebox. This limits the likelihood of the powder becoming airborne and subsequently coating internal surfaces excessively. Additionally, certain stages of ANFD operation occur under vacuum conditions which means utility lines (i.e. dust filters) need protection to avoid recirculating contamination downstream to ancillary systems. Similarly, there is an issue of cleanability. ANFD systems will offer limited flexibility if trace product retention is an issue, thus operators must be able to safely clean the vessel without risk of exposure to themselves.
At Powder Systems Limited, we design ANFD solutions with enclosures suitable for the most potent drugs in development. We can anticipate critical features that will elevate the filter drying process from both performance and regulatory perspectives.
One way we achieve this is by carefully designing the vessel to eliminate retention areas. The goal therein is to maximise cleanability and flexibility, creating a safer and more versatile instrument. Our dust filter is also designed with HPAPI production in mind, featuring a safe change design that blocks particles from going downstream.
Lastly, we also offer a preventative maintenance programme that maintains the performance of ANFD systems to ensure process optimisation and user safety. The first rule of containment is proper housekeeping, thus maintaining optical conditions is key to operator safety. Our goal is to help our customers meet the growing demand for high potency compounds and to extract the most value from this changing marketplace, without ever putting operators at risk of exposure to potent chemicals.
References
1. https://www.chemanager-online.com/en/news/rise-hpapi-molecules
2. https://www.pharmtech.com/view/understanding-containment

