The SCHOTT iQ platform has been designed to set new standards for the pharmaceutical industry. By providing SCHOTT’s high-quality containment solutions in a ready-to-use (RTU) packaging configuration, pharmaceutical companies can keep their fill-and-finish operations as simple as possible. SCHOTT iQ consists of three distinct primary packaging types.
syriQ prefillable syringes: designed to meet the stringent and evolving needs of the pharmaceutical and biotech industries, these glass syringes are manufactured on fully automated production lines and are washed, siliconised, assembled with a Luer lock and tip cap closure or needle shield, and supplied ready for the fill-and-finish process.
cartriQ is SCHOTT’s platform of ready-to-use cartridges for pen and autoinjectors, as well as wearable drug delivery devices. It’s design ensures smooth processing on fill-and-finish lines, widespread device compatibility and reliable performance during administration. With state-of-the-art FIOLAX borosilicate glass, baked-on silicone and high quality rubber components, it’s is the ideal containment solution for sensitive biologic drug formulations.
adaptiQ ready-to-use vials are prewashed and presterilised with state-of-the-art industry processes and can be filled on a wide range of existing and new fill-and-finish lines. They come in three distinct packaging options — the tray, the cup nest and the clip nest — and are available in sizes from 2R to 50R. They are also pretested with nested closure systems.All products within the SCHOTT iQ platform are compliant with ISO 11040-7 — the decade-proven market standard for RTU syringes. More specifically, on a container type level, syriQ is compliant with ISO 11040-7 and cartriQ is compliant with ISO 21881 (Figure 1).

Figure 1: Three distinct types of compliant primary packaging
The adherence to proven and industry wide accepted standards represents the solid foundation underpinning the benefits of the SCHOTT iQ platform. Three main areas of benefit stand out: reduced complexity, maximum flexibility and enhanced patient safety.
Reduced complexity
SCHOTT iQ reduces the complexity of traditional filling processes. Washing and siliconisation machines as well as heat tunnels are no longer needed as the ready-to-use containers already come presterilised. There is also a significant reduction in cleanroom space and, as such, in the overall total cost of ownership.
The company’s holistic approach standardises the industry’s trusted nest-and-tub format for ready-to-use syringes, vials and cartridges. The containers are nested and placed in a three-inch ISO 11040-7 industry standard tub, covered with a Tyvek inlay and seal, then packed in a header bag. A second header bag supplies further protection. The product is then sent for sterilisation.
The use of pretested and standardised packaging can also reduce time-to-market by increasing flexibility to accommodate new drug development, clinical trials or scale-up to commercial production, as well as cutting the time required to install, commission, qualify and validate a new filling line.
Increased flexibility
The trends towards smaller batch sizes means that fill-and finish-operations need to be more flexible than ever. As different drugs require different container types, the SCHOTT iQ platform offers a choice of presterilised vials, syringes and cartridges.
The platform was developed in close collaboration with a number of industry leading component suppliers and filling line manufacturers to standardise the packaging and tub dimensions. With SCHOTT iQ, it’s possible to run multiple products in various containers on one flexible filling line.
All three products — syriQ (RTU syringes), cartriQ (RTU cartridges) and adaptiQ (RTU vials) — are packed in the same tub using the same nest format.
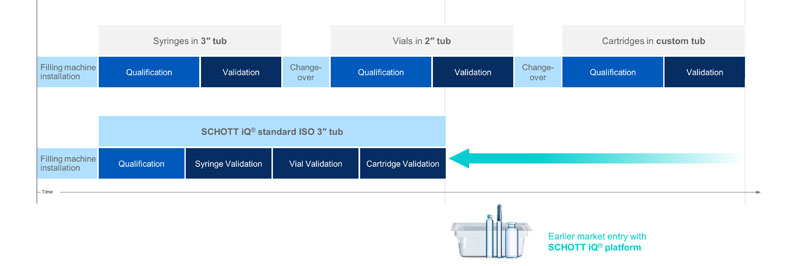
Figure 2: Standardised tub geometry and sequential validation accelerate time-to-market
Because the platform is based on the industry standard three-inch tub, qualification only has to be done once for all formats, followed by a sequential validation for all container types or formats (Figure 2). This accelerates the process, as changeover times and recurring qualifications can be omitted. Thus, having validated the three-inch tub, the container type can be changed easily, because the prevalidated tub is the same.
Enhanced patient safety
Patient safety is the most critical aspect of the pharmaceutical industry. SCHOTT iQ components are manufactured and processed in state-of-the-art, cleanroom environments, using scientifically selected high-quality materials.
Furthermore, thanks to the nest configuration, containers are individually held in place to avoid glass-to-glass contact, hence reducing scratches or micro-cracks. This not only reduces cosmetic defects but further reduces the risk of particle creation that could jeopardise patient safety.
The combination of high-quality drug containment solutions and the nested format provided by the SCHOTT iQ platform enables transport and filling processes that are free from glass-to-glass contact. This reduces cosmetic defects, fracture and breakage, as well as minimising particle levels.
One of the major benefits of SCHOTT iQ is that the high-quality containers are supplied in a washed and presterilised configuration. In the traditional bulk filling process chain, sterilisation is usually done at the depyrogenation step; the products are washed, dried and then passed through a heat tunnel to depyrogenate them at temperatures of 280–300 °C) to ensure they are sterile before they are introduced directly into the isolator.
In contrast, for the SCHOTT iQ platform, sterilisation is done after washing, drying, depyrogenation and denesting, and prior to the containers being transported to the aseptic fill-and-finish line (Figure 3).
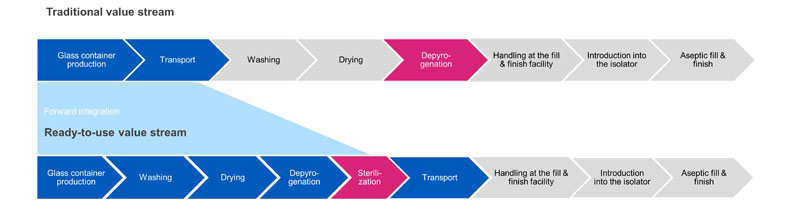
Figure 3: Traditional versus ready-to-use value streams
The sterilisation process for all SCHOTT iQ containers follows strict ISO requirements. All sites are certified by ISO 9001 and ISO 15378, and comply with Ph. Eur., USP and JP standards. Sterility is quantified with a sterility assurance level (SAL), which indicates that a product has been rendered sterile and remains so during its shelf-life.
Sterilisation is qualified and validated using biological indicators to ensure that the process has been done correctly. The SCHOTT iQ platform uses steam sterilisation for cartriQ cartridges and ethylene oxide sterilisation for adaptiQ vials and syriQ prefillable syringes.

