It’s unfortunate when a new drug product formulation is produced on a rotary tablet press for the first time and defective tablets result because the formulation sticks to the punch cup and the logo engravings on the tooling.
This situation often leads to a chain reaction involving the lead formulator, the clinical supply compression team, regulatory affairs, marketing and the tooling supplier. The parties discuss the issue, make changes to the formulation building process and make a few modifications to the tablet engraving cuts.
Another attempt is then made to produce tablets that meet the required physical properties and dissolution criteria without the previously encountered sticking and picking issues. If all goes to plan, the next tablet production run yields acceptable results on a small-scale tablet press.
Collecting helpful data
To avoid production-related difficulties, the development team should consider what data is needed during the tablet making process to ensure that scale-up is successful. A lack of data acquisition early in the development process can lead to all kinds of issues noone wants to take to the product team.
Was a study done to determine whether tablet tensile strength increased with rising compression force? And were these study results linear within the range required to produce tablets that meet product quality parameters?
The answers to such questions identify whether there is a compression force range where tablet strength may actually decrease with increasing compression due to the deformation characteristics of the API or selected excipients. A plot of compression force against the tablet tensile strength should be inspected. If the tablet tensile strength is lower at high compaction forces, there may be trouble when the process is scaled up to a larger tablet press (Figure 1 shows plots for three tablet formulations: a, b, and c).
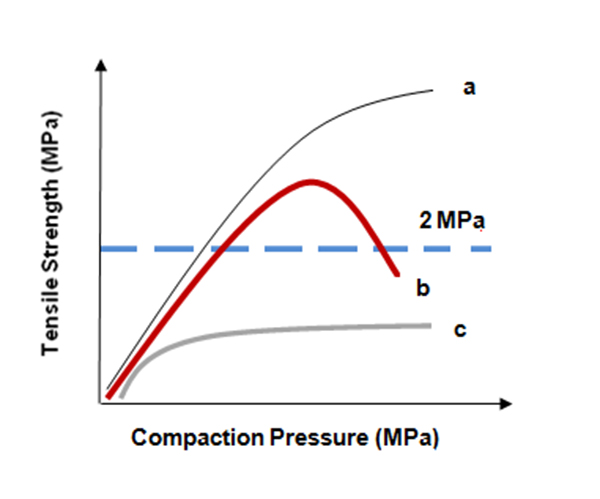
Figure 1. Tabletability profiles
If the formulation achieves clinical success, a larger tablet press will be necessary to meet the needs of future trials and market supply. Larger tablet presses have larger pitch circle diameters, therefore the consolidation/compression force profile on the larger tablet press will be different than on the smaller press.
By setting the compression force to a constant value while varying the speed of the tablet press, the strain rate sensitivity of the formulation can be assessed on a small scale. This test must be done carefully to ensure that the weight is adjusted to enable constant compression force at each press speed and tablet weight uniformity is maintained.
Plotting the turret tangential velocity against the tablet tensile strength should result in a reasonably straight horizontal line (Figure 2). A straight line with a significant negative slope is the characteristic of a strain rate sensitive powder that will be problematic as the press speeds up for higher production.
The faster speed on a larger tablet press reduces the dwell time of the compaction and sensitivity to dwell time may be difficult to address if the formulation has been registered and changes are not possible. Compensation for this problem is often a matter of increased compression force – an acceptable solution unless the formulation has already illustrated a non-linear relationship with respect to tensile strength and increased compression force. A possible last resort can be to utilise a different punch head profile with an increased head flat.
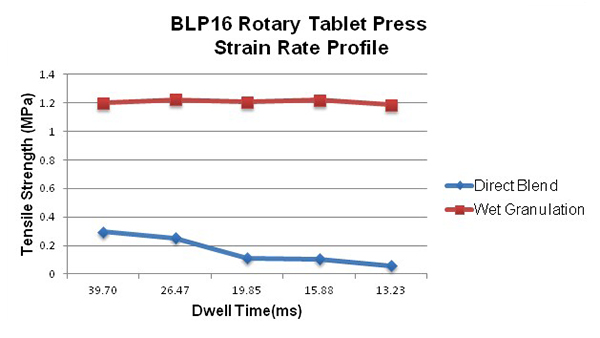
Figure 2. Strain rate sensitivity
Collect data but don’t forget the tooling
After good data has been collected, it is imperative to remember to keep tooling in good working order. Once the formulation is properly characterised and scale-up is successful, life is good – until the manufacturing unit calls with the report that the fourth lot is sticking on the lower punches and tablet defects are rampant.
“Now what?” is the cry of the product team. What does the compression tooling look like? What is the condition of the punch cups and their surfaces? How are the punch cups polished?
While tool polishing procedures vary from company to company, inappropriate use of nylon brushes, diamond paste and cotton bobs with high speed handheld drills can lead to suboptimal results. These methods must be used in a very conservative manner to avoid introducing microscratches and digs into the punch cup surface. An un-sewn cotton wheel on a high-speed buffing machine with polishing compound is the easiest and fastest way to buff the cup to a high gloss. It only takes a few seconds for each punch.
In some circumstances, the formulation causing problems may contain a micronized API with an average particle size in the low microns. Even in granulated formulations, small particles like this can become lodged in small voids or scratches in the cup and begin to accumulate. This leads to a haze in the cup and ultimately to sticking.
Minimising API while maximising data
It’s clear that in tablet development and manufacturing one thing can lead to another. Some formulations are forgiving but many new product recipes are not. Acquiring data early in the formulation process is key to recognising and avoiding likely problems at a later stage. Early formulation development is a fight for grams of API. A good practice to follow is to develop and validate formulation testing procedures on a small rotary R&D press that utilises minimal amounts of powder and maximises the data generated. Using software such as Natoli’s AIM™ Data Acquisition and Analytical Software for tablet press data acquisition can help save valuable time and API during the development process. Not paying attention to the number of turret rotations during early-phase testing can be the difference between getting the data you desire with 200 tablets as opposed to 2,000.
