Inhalation delivery has gained wide acceptance as an effective, non-invasive method for the local and systemic therapeutic delivery of active pharmaceutical ingredients (APIs).
The unique features of the lung — large surface area, thin alveolar capillary membrane, low enzymatic activity and avoidance of first-pass metabolism — are key elements of the efficacy of this delivery system.1 There are three main platforms associated with drug delivery via inhalation: nebulisers, metered dose inhalers (MDIs) and dry powder inhalers (DPIs).
Capsule-based inhalers belong to the category of dry powder inhalers and work according to a simple two-step procedure. Capsules are punctured or opened by pressing the device buttons. Then, after inspiration, powder is absorbed into the lung pathway (Figure 1).2
The purpose of this article is to describe the scenarios in which the application of these devices is especially convenient for patient use, formulations and drug delivery.
High-dose drugs
Dry powder inhalation has become a very attractive option for pulmonary drug delivery to treat diseases such as cystic fibrosis and its associated lung infections. Compared with traditional pulmonary drugs for asthma and chronic obstructive pulmonary disease, these therapies require the administration of higher doses to the lung.
Capsule-based inhalers provide a convenient platform in these situations as they can deliver doses of up to 100 mg. This is a significant advantage compared with MDIs that can deliver 1 mg or blister-based inhalers that release approximately 10 mg.
One example is Colobreathe from Forest Laboratories (Figure 2), which was approved in 2012 by the European Medicines Agency (EMA). This is a dry powder formulation of colistimethate sodium that’s administered using a Turbospin DPI (capsule-based inhaler).

Figure 2: The Colobreathe Turbospin inhaler
One capsule contains 125 mg of drug to be inhaled twice a day. Furthermore, positive results were recently demonstrated in a clinical dose escalating study (16–80 mg) for Aerovanc (Savara Inc.) for a dry powder formulation of Vancomycin in capsule-based inhalers.3
It’s the first inhaled antibiotic to be developed for the treatment of respiratory methicillin-resistant Staphylococcus aureus (MRSA) infection in patients with cystic fibrosis.4
Developing generic drugs
Generic orally inhaled products (OIPs) must follow the rules of therapeutic equivalence, with reproducibility being key. The delivered dose, fine particle dose and fine particle fraction of the inhaled API must mimic the branded product.
Plus, generic products must replicate the aerosol particle size distribution of the referenced OIP in aerodynamic assessments with certified multistage impactors.
As the regulations do not require the construction of inhalers for the original and generic OIPs to be identical, only the internal aerodynamic resistance must be similar (±15%) to the original product inhaler. Complicated multidose devices are often replaced in generic applications by simpler and cheaper single-dosage (capsule) DPIs.
Capsule-based inhalers offer the potential to change the parameters and obtain different results for the particle size distribution in a quick and efficient way.
Parameters such as inhaler design, capsule type, lactose particle size distribution or mixing can be modified to obtain different results in the aerosolisation process, as needed. An interesting example is Diskus, a fluticasone/salbutamol mixture originally developed in blister-based DPIs (Figure 3). A generic version of this product has been developed in capsule-based inhalers.5
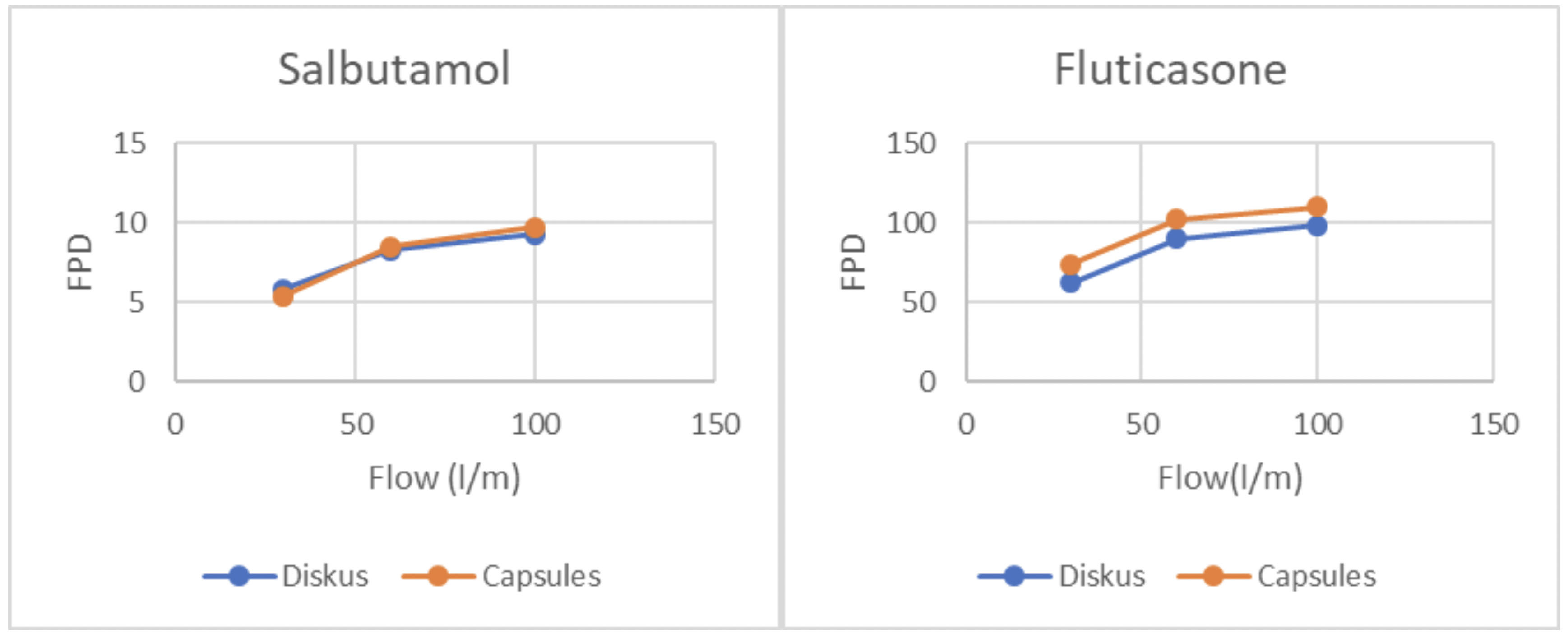
Figure 3: Development of the generic Diskus capsule-based inhaler. Fine particle dose versus flow rate
Moisture-sensitive products and amorphous powders
For inhalation drug delivery, amorphous powder formulations offer key benefits for drug development. Increasing the bioavailability of poorly soluble drugs, improving the biochemical stability of biologics and expanding delivery options using various drug combinations are assets.
Conversely, amorphous formulations usually have poor physicochemical stability. Such solids are thermodynamically unstable and can undergo crystallisation on storage.
Product has the tendency to crystallise with increased humidity as water plasticises to become solid by lowering the glass transition temperature.
Although macromolecules such as proteins and polypeptides are not easily crystallised, any amorphous small molecule excipients present in a formulation can more easily become crystals. Additionally, moisture may affect powder dispersion through capillary force.1
Capsule-based inhalers can overcome this problem. Hypromellose (HPMC) capsules benefit from a low moisture content (3–8%), which can be reduced further by equilibrating them at a low relative humidity (RH) after filling.
The capsules are then packaged in coated aluminium blisters, which minimises moisture exposure during storage. As there is a direct correlation between capsule moisture content and RH, storing the capsules at a low RH reduces the moisture content (Figure 4).6
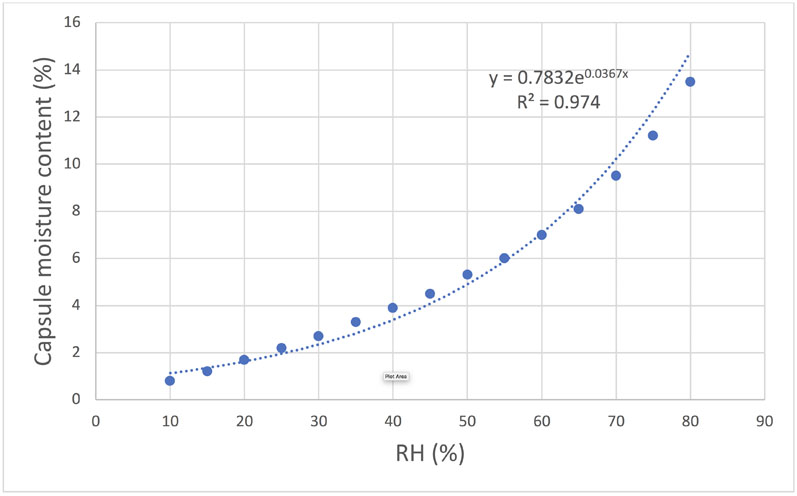
Figure 4: Correlation between relative humidity and moisture content for HPMC capsules
Compared with gelatin, HPMC capsules have a higher tolerability against fracture at low RH conditions. Gelatin capsules can become brittle in a low RH environment as the integral water acts as a plasticiser.
Should capsule fracturing occur in the inhaler when punctured, issues regarding getting the full dose into the patient’s lung may occur. One example that illustrates the ability of HPMC capsules to increase the stability of an amorphous powder is the Braltus product.
This comprises amorphous tiotropium in an HPMC capsule-based inhaler. This amorphous tiotropium has a higher bioavailability than the standard version (Spiriva): a 14 µg dose of Braltus is therapeutically equivalent to 22 µg of Spiriva.
References
- I. Chen, et al., “Amorphous Powder for Inhalation Drug Delivery,” Adv. Drug. Del. News 100, 102–115 (2016).
- Novartis, Utibron Neohaler: Instructions for Use (www.sunovionprofile.com).
- S. Claus, et al., “How Can We Bring High Doses to the Lungs?” European Journal of Pharmaceutics and Biopharmaceutics 86, 1–6 (2014).
- Colobreathe: www.medicines.org.uk/emc/product/3063/smpc.
- A. Hejduk, et al., “Technical Challenges in Obtaining an Optimized Powder/DPI Combination for Inhalation Delivery of a Bicomponent Generic Drug,” Journal of Drug Delivery Science and Technology 44, 406–414 (2018).
- J. Kalafat, et al., “The Science Behind Capsule-Based Dry Powder Inhalation Technology,” On Drug Delivery 80, 6–8 (2017).
