Most medicines rely on regular, repeated dosing to achieve their optimum effectiveness and the best possible outcome for the patient. But to ensure that patients take the right dose at the right time, pharma companies need to make it as easy as possible for them to comply with the regimen.
The barriers to patient compliance are myriad, and each and every therapy area or disease state presents its own challenges, points out Austin Fido, account director at US-based consultancy One World DMG. ‘A patient population being treated for cognitive problems has potentially different compliance problems from patients being treated for restricted motor skills.’
Two recurring themes in the compliance discussion are education and practical considerations. Patients don’t always fully understand why they should be taking their medication on the schedule dictated by their prescription, and if they are don’t understand the importance of adherence to the regimen they are less likely to comply with it.
On the practical front, having to take a pill is another thing to remember. If a patient has more than one pill to take, that’s more than one thing to remember, says Fido. ‘Busy and complex lives are made busier and more complex by dosing schedules and managing the requirements of varying prescriptions.’
A key challenge for the industry is that compliance rates vary greatly according to the condition being treated, says Steve Kemp, business development director, Brecon Pharmaceuticals. The origins of compliance-enhancing packaging can be traced to the introduction of the birth control pill almost 50 years ago: today, this category of product boasts the highest rate of compliance – over 95% – because users understand that non-compliance can have a very rapid and tangible impact on their lives.
At the other end of the scale, compliance rates for chronic conditions such as congestive heart failure, diabetes and glaucoma, where disease progression may be more insidious, languish at around 42% – although in the case of glaucoma, this increases to 58% once sight has been lost in one eye.
The calendar blisterpack is still the ‘front line of defence’ in compliance packaging, says Kemp, providing a straightforward yes/no indication of whether a medication has been taken on a particular day or at a particular time. And studies repeatedly show that the blister – with or without calendar – delivers greatly improved compliance for solid dose products compared with alternative pack forms such as bottles.
Typical of these is a 2005 study that measured the relative compliance and effectiveness of the ACE inhibitor lisinopril supplied in walleted blisterpacks as against bottles. Results showed that 48% of patients receiving blisterpacks saw improved blood pressure compared with less than 20% for those receiving bottles.
The compliance-enhancing benefits of the blister can be complemented by combining it with other pack strategies: patients are far more likely to comply with their regimen if they understand what they have been prescribed and why. The wallet pack used in the lisinopril study served to ensure that product and dosage information remained visible and attached to the blister at all times.
Correctly executed, compliance-enhancing packaging can make a huge contribution to patient adherence, says Kemp. ‘It offers visual confirmation of daily doses taken, gives a clear indication of when a new course is required and serves to remind the patient of the reasons for treatment and the importance of the drug in his care plan.
‘There is also much that can be learned from packaging designed for clinical trials supplies, where compliance is essential to the reliable outcome of any particular study and therefore closely supervised.’
Pharmacists have become an increasingly important source of trusted information for patients and are therefore in a good position to address the thorny issue of patient adherence, states Dr David Spackman, European sales director, adherence and delivery systems at MWV Healthcare.
next blockbuster
‘There is a growing belief that patient adherence is perhaps the next blockbuster for pharmaceuticals – if you can make sure that the patient takes the medication correctly and gets the best outcome, then not only will the relationship with the doctor be good because the patient feels he is getting something that is doing him good, but the doctor will also be more likely to prescribe that medication in future, which will benefit the pharmaceutical company,’ he explains.
‘Payers are now saying that they want to pay for outcomes, not treatment, and this is changing fundamentally the paradigm in the industry about how important it is for patients to take the medication correctly. If they don’t take it correctly, they will almost certainly not get the outcome that the payer is prepared to pay for.’
When a patient receives his medication from the pharmacist, he will often be given the drug in a blisterpack, inserted into a carton together with a leaflet. Three out of those four elements are packaging-related, emphasising the influence that packaging can have on the patient experience of the drug.
‘For years it has always been about the drug, but now the pharmaceutical companies are beginning to see it is the confluence of all those things that will be a major aid driving patient adherence,’ asserts Spackman. ‘It is possible for pharmaceutical companies to have a real conversation with their patients through packaging.’
The term ‘packaging’ in this context may, in itself, be unhelpful, Fido believes, as it generally connotes a fairly basic requirement: to get a product safely from the manufacturer to the consumer. In most cases, packaging is disposed of once that job is done.
‘When we start to talk about using pharmaceutical packaging to address the patient compliance issue, we are asking packaging to step beyond its traditional role, he suggests. ‘Not to assume airs and graces, but we are basically talking about drug delivery systems: more comparable to the auto-injection pens that are increasingly widely used for biologics than the traditional amber pill bottle.’
There are a number of ways in which packaging design can make the taking of a medicine an easier, more convenient and more pleasant experience for the patient.
MWV Healthcare recently carried out some research among more than 100 pharmacists in the UK to assess their views on preservative versus non-preservative formulations for products dispensed through nasal sprays, such as decongestants.
Most decongestants contain a preservative to keep them free of bacterial contamination during the lifetime of the product, but these preservatives can irritate the nasal mucosa and cause an unpleasant stinging sensation, and even cause rhinitis medicamentosa, or rebound nasal congestion.
downward spiral
‘For patients to get the best out of the medication they need to take it for the correct period of time, otherwise the outcome is not good and the payer’s money is wasted. So rather than getting a virtuous circle of benefits for all parties concerned, there is a spiral downwards in which money gets wasted and the patient is dissatisfied with the outcome overall,’ says Spackman.
The MWV research found that two out of three pharmacists surveyed already had a preference for preservative-free formulations, but this rose to four out of five once the benefits were explained to them. The survey data suggested strongly that pharmacists are more likely to recommend a preservative-free otc product than one with preservatives, he believes.
The company has designed a nasal spray pump – PFP N – that can be filled aseptically and dispenses an accurate, precise metered dose. When the pump is actuated, the internal pressure increases, causing a spring-loaded seal to open, expel the liquid into the nose and immediately shut off without any suck-back.
When the pump is at rest, the channel to the atmosphere is completely locked throughout the pump, preventing the ingress of contamination. The pump is available in a vented and non-vented version. The vented version has a 0.2µm filter that keeps out any organisms so that the product remains aseptic throughout its lifetime. A variety of colour-coded options can also be used for branding or to indicate different strengths of the drug, for example.
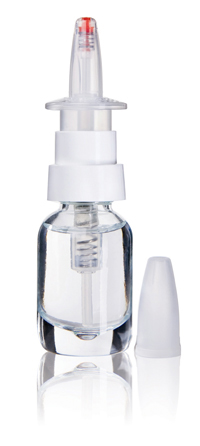
The PFP N can be filled aseptically and dispenses a precise metered dose
‘We think this provides brand owners and brand managers with a valuable way of differentiating their brand in the marketplace as well as giving real benefits to the patient,’ says Spackman. ‘Going forward, we think that many different drugs will look at the nasal route as an interesting way to administer new molecules that are not biologically available through the gut.’ Ophthalmological treatments are another area where preservative-free technology could be of particular interest.
In September 2009 MWV Healthcare launched Med-Easy, a slim, rectangular pack resembling a small book or diary that combines the blister, carton and patient information leaflet in a single unit. Based on research among patients on chronic medication across Europe, Med-Easy is portable and discreet and can be opened and closed with one hand. The cardboard folder protects the blister and can be provided with a tamper-evident, tear-off seal. The leaflet is attached to the inner cover using a fugitive adhesive, allowing it to be removed and replaced as required.
A further advantage is that Med-Easy can be run on existing standard cartoning lines with minor changes to the infeed. This means that it can be produced near to the market where the product is to be sold, shortening the supply chain and improving logistical control.
Improving patient compliance by providing greater convenience was also the motivation behind the Burgopak system, for which Brecon has the only high speed manufacturing line in Europe. ‘Burgopak is certainly one of the most important innovations we’ve seen in pharmaceutical packaging for many years,’ says Kemp. ‘Key to its appeal is the fact that the integrity of the pack is maintained at all times: this has already been seized on by the pharmaceutical marketing community as an excellent means of reinforcing brand messages in the highly competitive otc sector.
‘However, its compliance-enhancing capa-bilities are arousing significant interest from the prescription sectors too, particularly since its distinctive and patented design is in itself an effective deterrent for counterfeiters.’
integrated information
Essentially the Burgopak package presents its contents to users through an innovative belt-drive mechanism. The pack is opened smoothly by pulling on a tab, and pushing the tab closes it again. This action reveals a blisterpack and a panel that can hold literature inserts or other information.
Information about the drug is integrated into the packaging and therefore does not become separated from the pack. Single and double blister designs are available; in the double-blister version, the second blister slides out to reveal both the attached leaflet and the blister foil.

The Burgopak is opened smoothly with a pull on a tab to reveal the blister and a panel that holds literature inserts or other information
According to Kemp, its benefits go beyond just giving a brand a higher perceived value. It can become part of the product being sold, increases the amount of surface area that can be used for branding, creates a lasting impression on the end user, and can also improve customer safety. Burgopak was recently awarded the top US rating for its CRSF (child-resistant, senior-friendly) properties.
But the question remains: who should bear the cost burden of all this packaging innovation?
The problem is the more compliant-focused the package, the more expensive it is likely to be, and it is probable that many great ideas cannot get off the ground due to their high cost in a very price-conscious world.
‘If that question had been resolved, there would be a great deal more compliance packaging in circulation than there is today,’ says Fido. ‘At the moment, it is assumed the cost will be borne by the product manufacturers: the pharma companies.
‘And in a cost-constrained environment – and the likelihood that the pressure on cost will remain for some time to come – purchasers are not excited by solutions that are more expensive than their existing supply chain arrangements.’
cost of non-compliance
In the US estimates put the cost of poor patient compliance as high as US$290bn. That cost is drawn from a number of factors, including the cost of treatment for more severe conditions brought about by a patient’s failure to stay on a course of medication for a more minor concern. And with the debate in the US over healthcare costs in general, there is an argument for government and other healthcare payers (insurance companies) to provide incentives to patient compliance, Fido suggests.
‘In that context, the cost of well designed packaging may stand up well against the cost of other remedies to the compliance problem (more doctors; longer patient consultations; more intensive education efforts).
‘To continue that theme – if pharmaceutical companies were to start to look at compliance packaging in the context of other patient education efforts, e.g. websites, helplines and patient support networks, it may be that budgets would be well directed toward point-of-use solutions that help to minimise adverse events and patient confusion over what medications to take at what time and with what accompaniments.’
Spackman believes that the premium over the cost of a standard folding carton can be easily justified. For example, if a product is positioned number two or three in the market and is slightly less expensive than the brand leader, repositioning it by using a pack that is clearly preferred by consumers could secure a strong share of that premium market for a very slight increase in the cost of goods.
‘We have done some modelling around total cost of ownership and that has shown that you need only a very modest increase in sales to make this worthwhile,’ he says. ‘We think brand managers can get good ROI for choosing this solution.’
Similarly, Kemp does not believe that the cost should be passed on to the patient as it is the drug manufacturers who stand to benefit financially in the long run. ‘As the pharmaceutical companies gain greater compliance for their drugs, the more packs they are likely to see, which in turn will result in a significant financial benefit to the manufacturers,’ he points out.
‘Another contender to foot the bill would be private medical insurers: if patients, through the use of this new packaging, were taking their medicines in the correct manner, and therefore not having to be re-admitted for further treatment, in the long run the insurers would also benefit from a reduced outlay.’
In terms of increasing compliance, improving patient safety and preventing medication errors, a well-designed pack can pay for itself many times over.

