Change control and variation management are processes for which co-ordination between functional areas is critical to a company’s agility, profits and compliance. Yet, there are information gaps that hinder good collaboration. When a company wants to change an approved product, manufacturing process or supplier, it involves a complex and lengthy procedure spanning multiple departments. It’s a challenge in frequency and scale.
Numerous changes: As an example, a top 20 pharma company with more than 40,000 change requests may end up with approximately 15,000 changes that must be implemented across the organisation.
Lengthy cycle times: From initial assessment to final regulatory approval and implementation, changes can take several months, or even years, to complete.1 The change-control review board is dependent on information from other teams — such as regulatory, manufacturing and supply chain — to make informed “go” and “no-go” decisions on requested changes. Often, however, information provision is significantly delayed because of a lack of visibility and the knowledge to reliably assess the impact of a change in other areas, including suppliers and external partners.
In this article, we’ll focus on how to improve the role of regulatory within the change-control process so that the quality team or change-control review board can make informed decisions quickly and speed submissions (Figure 1).
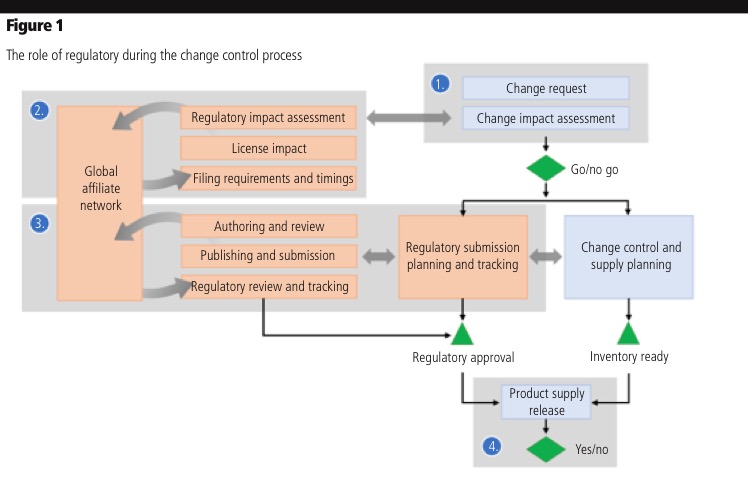
Making go/no-go decisions on proposed changes
Assessing the implications of a change request can take several weeks, or even months, owing to information gaps across functions and locations. Regulatory can speed performance along three parts of the process: identifying impacted product licenses, determining the required variation filings and estimating the time and resources required to secure approvals.
Assessing when and where to file a variation: As process owners evaluate the impact of a change request, they need to determine which regions would be affected and the types of filings required at each location. Countries have different variation requirements, and there is room for interpretation as to whether a filing is needed. Making the wrong judgment results in extra time, effort and cost.
The industry has recognised the uncertainty and expense that companies face related to filing variations, and is working on new recommendations to manage post-approval CMC changes. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals in Human Use (ICH) has drafted the concept paper, Q12, which will be released for public comment as the next step forward. A main element of the initiative will be to define “established conditions” for changes that require regulatory approval upfront, at the time of the initial licensing approval. Low-risk changes will not be included, as established conditions will be documented — along with any other changes — in a company’s quality system.
ICH Q12 will also encourage manufacturers to define post-approval change management protocols (PACMPs) that describe how they will manage variations during a product’s lifecycle. Although PACMPs exist in the US and Europe, they have not been widely adopted. The ICH hopes to stimulate broader use; this is especially important when multiple specific changes are expected for a particular product. Establishing consistency will speed change and instil greater confidence in regulatory bodies about the way companies handle variations. With ICH Q12, firms establish the regulatory intelligence upfront to ensure they make informed decisions when filing variations.
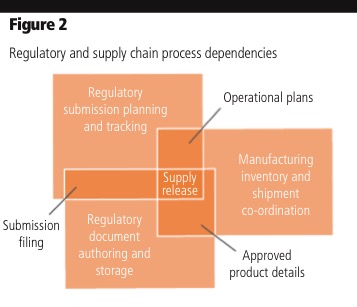
Determining the scope of impact for variation filings: It’s challenging to assess the extent of the change’s impact on the organisation. A single ingredient or manufacturing change can affect multiple products and every license under which they are marketed. In many cases, regulatory depends on quality and supply chain information to determine which products are impacted by a change (Figure 2). With product registrations in the RIM system, regulatory can quickly determine which products are affected by a change and where those products are licensed.
Regulatory teams must frequently contact affiliates for local licensing details. If affiliates maintain product licenses in a shared global system, the team at headquarters can quickly run an impact assessment report across geographies. Also, headquarters can gather additional data as needed via trackable workflow tasks. This helps to address one of the most common delays in the assessment process, by making it easier to gather information from affiliates and providing visibility into outstanding requests.
Evaluating the scale of a proposed change: Once regulatory knows which products and licenses are affected, they must estimate the amount of time and effort required to execute the proposed change. Compiling this information can require multiple phone calls, emails and searches through spreadsheets, often generating more paper documentation in the process. There is an opportunity to capture responses systematically and globally from regulatory professionals during impact assessments.
Some countries restrict sponsors to one active submission at a time. Tracking this information globally provides visibility into ongoing submissions, so expected delays can be incorporated into the broader decision making process. It also provides an historical view into the length of time it took to make past filings in different markets.
Regional affiliates may also have visibility into other activities related to the product that must be considered. If they are using a global RIM system as part of their regular processes, that information is more likely to surface in a report. With affiliates directly inputting data into the global system, headquarters can easily see if they have information for all countries or markets and estimate the resources and time it will take for a change to be implemented.
Making an informed cost/benefit decision: In addition to identifying criteria associated with a proposed change, regulatory should provide cost estimates. A change that may initially seem like upfront savings to the company can end up costing more in the end, owing to high levels of effort and expense incurred by the teams and regions filing the variation and implementing associated changes.
The more companies consolidate information and provide greater transparency, the greater insight individuals have throughout the process. During the assessment, teams can more easily evaluate data from the RIM system and extrapolate that into cost calculations to help inform a go/no-go decision.
Reference
- S. DeMare, “The Future of Post-Approval Change Management,” http://virtualregulatorysolutions.com/the-future-of-post-approval-change-management (December 2016).
