Although they typically function through silencing mechanisms, as in antisense oligonucleotides (ASOs) and small-interfering RNAs (siRNAs), we are beginning to see the emergence of activating oligonucleotide therapeutics.
Regardless of their specific mechanism of action, the greatest hindrance to the widespread use of oligonucleotide therapeutics has been their inability to be precisely and efficiently delivered to the target tissue and effectively internalised into cells to reach their molecular targets in the cytosol or nucleus.
Evolutionary defences designed to keep invading RNAs from penetrating cells have meant that since the start of the oligonucleotide therapeutic revolution, the problem to solve has remained the same: delivery. Indeed, all other issues with developing oligonucleotides as a therapeutic modality have paled in comparison with the delivery problem.1
The major approaches to overcome this have included loading oligonucleotide therapeutics into lipid nanoparticles, conjugation to GalNAc for hepatic delivery and conjugation to specific affinity ligands for biomarker-targeted delivery.
Engineered lipid nanoparticles for selectivity
Lipid nanoparticles (LNPs) are chemically synthesised, multicomponent lipid formulations (approx. 100 nm in size) that encapsulate oligonucleotide therapeutics for delivery to the target tissue.
Made famous by the COVID-19 vaccines from Pfizer-BioNTech and Moderna, LNPs protect the oligonucleotide molecules — such as mRNA in the COVID-19 vaccines — against degradation by ubiquitous nucleases whilst travelling to the intracellular site of action.
Ionisable lipids are positive at acidic pH levels and neutral within the blood. The use of ionisable lipids within the LNP structure binds the negatively charged backbone of oligonucleotide therapeutics for incorporation into the particle structures and prevents toxicity from cationic lipids once present in vivo.
LNPs injected intravenously invariably accumulate within the liver. Despite ongoing challenges in targeting LNPs to alternative tissues and organs, progress in particle formulation is being made that allows alternative tissue targeting.

Tuning LNPs for organ selectivity has included the use of various polyethylene glycol (PEG) and PEG-alternatives. PEG is typically incorporated into oligonucleotide therapeutics to extend serum half-life. By varying the length and structure of PEG molecules, the charge of the various lipids and proportions of lipid components in LNPs has been shown to allow targeting away from the liver to alternative organs such as the spleen and lungs.2,3
A second strategy to target LNP biodistribution involves coating the particles with affinity ligands for specific biomarkers to promote uptake to selected tissues and organs. This approach uses antibodies and has been successful for LNP delivery to inflammatory leukocytes and epidermal growth factor receptor-positive tumour cells to treat inflammatory bowel disease and cancer, respectively.
Owing to the large size of antibodies and their propensity to raise immunogenic reactions, there is increasing interest in the use of alternative affinity ligands, such as aptamers, to target LNPs. Preliminary experiments using aptamers have shown promise in applications that cross the blood-brain barrier and/or target bone.4,5
Despite excellent progress in LNP formulation and emerging methods to selectively target these particles, the reliable production of consistent LNPs remains a challenge. Indeed, manufacturing the raw materials to make the particles was a limiting factor in during the production of COVID-19 vaccines.6
A direct line to the liver with GalNAc
GalNAc conjugation is an effective and highly reliable liver-targeted delivery platform for oligonucleotide therapies. The development of GalNAc (N-acetylgalactosamine) conjugates for the targeted delivery to the liver has enabled great improvements in the potency of oligonucleotide therapeutics through effective hepatic targeting.
GalNAc conjugation enables oligonucleotides to overcome the body’s defence system that prevents invading RNAs from getting inside cells.1 This approach is applied to allow for the efficient delivery of oligonucleotide therapeutics including ASOs and siRNAs into specific tissues and cells.
Tris-GalNAc binds to the asialoglycoprotein receptor — which is highly expressed on hepatocytes — resulting in rapid endocytosis.1 The use of GalNAc to target oligonucleotide therapeutics to the liver increases hepatic uptake by 7–10-fold compared with controls.1,7
Oligonucleotides administered systemically naturally distribute to the liver and other tissues, raising the question: how effective is GalNAc? This was addressed by a key study in 2014 that examined the cellular distribution in mice treated with non-conjugated or GalNAc-conjugated ASOs.
This study showed unconjugated ASOs were taken up predominantly by a non-parenchymal cellular fraction of the liver (>70%), whereas GalNAc-conjugated ASOs were predominantly take up by the hepatocyte fraction of the liver (>80%).8
The observed targeting was associated with an increased potency of the ASO, which has been repeated in many studies in the clinic, showing a 10–30-fold lower dose required for functional target knockdown with GalNAC conjugated ASOs compared with non-conjugated controls.7–9
Several GalNAc oligonucleotide conjugates are in clinical development (Figure 1) with many more in the discovery and preclinical pipeline. In addition, GalNAc oligonucleotide conjugates Givlaari (givosiran), Oxlumo (lumasiran) and Leqvio (inclisiran) have gained approval from regulatory authorities for the treatment of acute hepatic porphyria, primary hyperoxaluria type I and atherosclerotic cardiovascular disease, respectively.
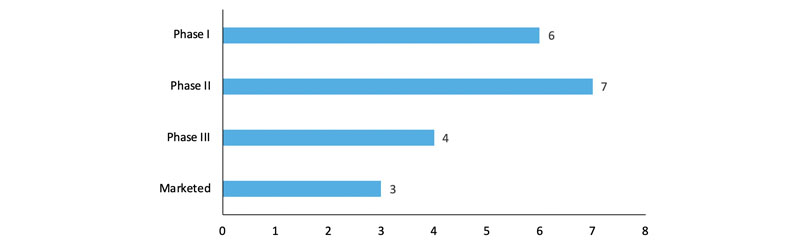
Figure 1: GalNAc-conjugated oligonucleotides in clinical development phases
An additional benefit of utilising GalNAc with oligonucleotide therapeutics is that the triantennary ligand can be incorporated into standard solid phase synthesis manufacturing methods, ensuring high consistency and expediency.10
Affinity targeting oligonucleotide therapeutics
Affinity targeting has been widely used in the development of cancer treatments to target toxic payloads as antibody-drug conjugates (ADCs) and novel format conjugates, with 13 ADCs approved for use as of September 2022.11
The change to the delivery of oligonucleotide therapeutics is a logical extension of chemotherapeutics in this growing field. Taking learnings from ADC development and manufacturability has accelerated the affinity delivery of oligonucleotide therapeutics.
The first molecule to enter the clinic came last year from Avidity Biosciences, an antibody-oligonucleotide conjugate that targets siRNA to muscle for the treatment of myotonic dystrophy type I, a rare muscle disease.12 However, the US FDA has recently placed a partial clinical hold on further enrolment owing to an adverse event in one of the trial patients.13
The reliance on antibodies for delivery brings substantial complications in manufacturing. Additionally, the large bulky size of antibodies ties developers to relatively high drug dose regimens. It is anticipated that using smaller ligands could help to overcome these challenges.
Examples of such are now entering the clinic, with Dyne Therapeutics using Fab fragments for the affinity based targeting of oligonucleotides to muscle, and Aro Biotherapeutics using a protein-based antibody alternative (Centyrin) to target siRNA to muscle.14,15
An alternative to antibodies
Judy Lieberman from Harvard Medical School developed one of the first oligonucleotide conjugates utilising Fab fragments in 2005.16 She has now moved to working with RNA aptamers for affinity targeting.17
As oligonucleotide molecules themselves, aptamers offer the binding properties of antibodies without the potential immunogenicity and manufacturing liabilities of an antibody based conjugate.
They can be synthesised as single molecules with the oligonucleotide therapeutic in place, to make the most of the consistent solid phase synthesis manufacturing methods, or conjugated separately to allow for variation in the drug:aptamer ratio.
Aptamer Group works with many partners to explore the potential of aptamers as delivery vehicles for numerous therapeutics and conditions.
Ultimately, the efficacy and safety of oligonucleotide therapeutics is no longer the issue in question; problems of degradation and immunogenicity with these molecules were quickly dealt with in the early stages. Nonetheless, the challenge of targeted delivery remains.
The field is necessarily progressing multiple delivery options in parallel, with the potential for improvements on current solutions and the emergence of new solutions that could help target new tissues and resolve CMC challenges to overcome the current tissue issue.
References
- A.D. Springer and S.F. Dowdy, Nucleic Acid Ther. 18(3), 109–118 (2018).
- X. Hou, et al., Nat. Rev. Materials. 6, 1078–1094 (2021).
- H. Haas, presentation at the Oligonucleotide Therapeutics and Delivery Conference (21–22 September 2022, London, UK).
- R.M. Ray, et al., Belstein J. Org. Chem. 17, 891–907 (2021).
- C. Liang, et al., Nat. Med. 21(3), 288–294 (2015).
- www.scientificamerican.com/article/new-covid-vaccines-need-absurd-amounts-of-material-and-labor1/.
- A.J. Debacker, et al., Mol. Ther. 28(8), 1759–1771 (2020).
- T.P. Prakash, et al., Nucleic Acid Res. 42(13), 8796–8807 (2014).
- S.T. Crooke, et al., Nucleic Acid Res. 29(1), 16–32 (2019).
- I. Cedillo, et al., Molecules 22(8), 1356 (2017).
- www.bioprocessonline.com/doc/adcs-are-the-future-of-precision-chemotherapeutics-0001.
- https://clinicaltrials.gov/ct2/show/NCT05027269.
- www.neurologylive.com/view/fda-places-temporary-hold-trial-for-myotonic-dystrophy-agent-aoc-1001.
- www.dyne-tx.com/.
- www.arobiotx.com/pipeline.
- E. Song, et al., Nat. Biotech. 23(6), 709–717 (2005).
- Y. Zhang, et al., PNAS 118(9), e2022830118 (2021).
