Here, Dr David Bruchof, a Product Manager for Industrial Compressors at CompAir, offers five vital considerations that decision makers need to bear in mind when specifying a high-performance and long-lasting oil-free compressor solution.
Understand and assess the risks
Whether it’s laboratories, extruders, bottling plant, tablet presses or packaging processes, compressed air is used in a wide variety of pharmaceutical applications.
But, although the pharmaceutical industry is one of the most regulated in the world, it pays comparatively little attention to compressed air usage and the standards that air should meet when used in these environments.
Even though production processes are controlled by good manufacturing practice (GMP) guidelines, the European Pharmacopoeia, the US Food and Drug Administration and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, their scope on compressed air is fairly limited.
For instance, the European Pharmacopoeia only focuses on air for medical applications and, in this context, only makes a distinction in respect of artificial air.
There are potential risks regarding the purity of compressed air as, in many processes, it will often come into direct contact with products. This includes drying equipment parts, transporting substances through tubes and when compressed air is used as a pressure blanket for solutions in tanks.
Compressed air is also used for equipment control purposes in manufacturing production lines, such as pneumatically opening valves. And with many of these operating around the clock, it’s no surprise that making sure the right compressor is specified is essential.
At the start of a compressor specification, risks should be assessed and analysed. These might include the following:
- does the compressed air application involve direct or indirect contact with products?
- what is the volume of compressed air required?
- are the manufactured goods classified as a sterile medicine or microbiologically unstable?
- what potential contaminants from a compressed air system, such as particles or oil, might need to be considered?
- is the product being manufactured sensitive to moisture?
Consider potential contaminants
The intake air for a compressor is susceptible to contamination from almost any kind of particles, from pollen, dust and hydrocarbons to heavy metals such as lead, cadmium or mercury.
One important factor to consider is how particle concentrations in a system increase when compressed air is generated. And there is the very real danger that blow-off air from a pneumatic system may come into direct contact with a product.
Of course, any form of compressed air will naturally contain particles of contaminants. But, owing to the lack of regulations that define compressed air usage in these environments, manufacturers and pharmaceutical businesses need to establish the standards that compressed air needs to meet to be suitable for manufacturing processes.
The risk of contamination and its consequences should not be understated. Even the smallest possible risk of contamination can impair processes such as material handling, process air and product drying, and harm air curtains, control valves, cylinders and other tools.
These adverse effects can often only be resolved at significant expense, burdening factory owners and operators with costs that could otherwise be avoided.

Oil-lubricated or oil-free?
Many sites use oil-lubricated compressors. These use oil in the compression chamber to cool and seal the compression process. In many cases, these compressors provide an acceptable level of compressed air when manufacturing pharmaceutical goods.
However, they do rely on filtration systems such oil separator elements and downstream filters, which need to be periodically replaced. This, of course, adds to ongoing maintenance costs.
As a result, many facilities are now opting for oil-free technologies. Their very design centres around keeping oil particles out of a compressor and can offer a whole host of benefits, particularly for medical manufacturing sites.
For the highest standards of air purity, an oil-free compressor delivers! The technology can help energy managers to make valuable cost savings, plus improve operational efficiencies too. Whole life costs are reduced, with businesses being able to save on the cost of oil replacement.
Unlike oil-lubricated systems that require oil change intervals throughout their service life, there is also no need to purchase equipment that cleans and separates the oil from air, such as oil separators, filtration equipment and condensate treatment.
Finally, with so many businesses today facing ambitious sustainability goals, an oil-free option can play a valuable role in contributing towards a facility’s lower environmental impact.
Think long-term to choose the right technology
When choosing an oil-free compressed air system, there are a number of different technologies to choose from. Whether it’s scroll technology, which consists of an orbiting, rotating scroll and fixed scroll housing, which together create compression chambers, or water-injected rotary screw products, which use water to lubricate, seal and cool the compression process, the best solution will depend on a site’s needs and demands.
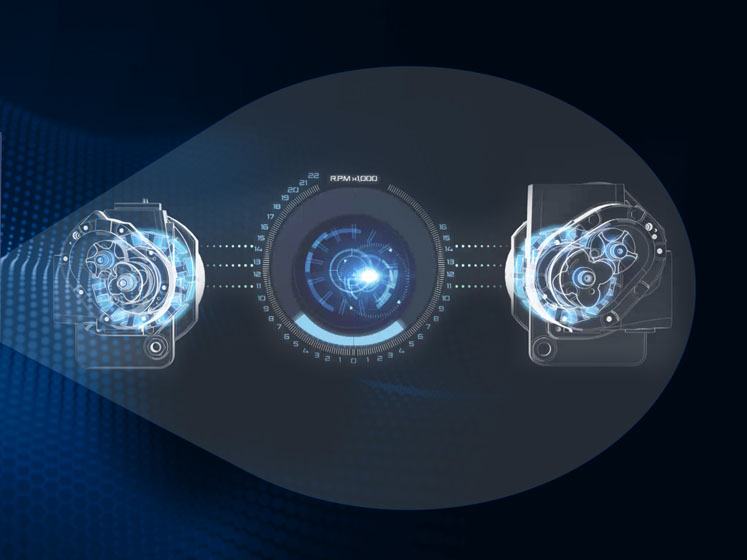
Featuring two highly efficient, permanent magnetic motors that replace the traditional gearbox set‐up, these variable‐speed motors can achieve speeds of up to 22,000 RPM and efficiencies greater than IE4.
Traditional models rely on a gearbox, which creates friction and results in efficiency losses, while also requiring high volumes of oil to lubricate the gears.
In contrast, Ultima’s motors directly drive the airend without the need for a gearbox, continuously monitoring and adjusting the speed of each airend. This ensures maximum efficiency and pressure ratios at all times.
Heat recovery offers further potential to save money and realise efficiency gains. More than 90% of the power consumption of a compressor is typically converted to heat. This waste heat can then be recovered to generate hot water at up to 85 °C.
This can be used, for example, as process heat in manufacturing operations. This is a feature that can help businesses to significantly reduce their energy costs.
Make maintenance matter
Finally, once the investment in a new compressed air system has been made, and the system has been installed, it is vital that owners and operators ensure it receives proper servicing and maintenance.
The latest compressors are also supported by Industry 4.0 solutions for compressed air analysis and proactive maintenance.
This enables users to control and optimise their compressed air processes, while continuous condition monitoring helps prevent unplanned system downtime during pharmaceutical manufacturing. It can therefore play a key role in avoiding any losses on a production batch.
