Biotechnology is providing new pathways to treatments for many uncommon yet life-threatening diseases. Dr Sarah Houlton looks at some promising candidates.
Although blood cancers are not uncommon, the number of different, distinct subtypes means that many of the drugs being developed to treat them have been given orphan designation – that is, they are designed to treat a disease whose incidence is less than five people in 10,000. In particular, numerous different biological medicines are under development, and the following biologics are some of those that have received orphan designation in the EU in the past few years.
Acute myeloid leukaemia is a cancer of the white blood cells. The bone marrow of AML patients makes large numbers of abnormal immature blood cells, or blasts, that accumulate in the bone marrow and hinder the production of normal blood cells. The disease is far more common in adults than children, and it affects fewer than two in 10,000 people in the EU. There are several subtypes, all of which progress rapidly, and five-year survival can be as low as 15%. Patients are typically given chemotherapy, and if that does not work, a bone marrow stem cell transplant.
The approach being taken by French company LTKFarma involves using human allogeneic T cells taken from a matched donor. One of the problems with a stem cell transplant is the potential for graft-versus-host disease (in which the newly transplanted material attacks the transplant recipient’s body) to develop; LTK’s treatment, TK54, is designed to overcome this problem.
The donor cells are modified to introduce a ‘suicide’ gene that encodes for the thymidine kinase, or TK, viral enzyme from herpes simplex virus type 1. This renders the dividing cells susceptible to the antiviral agent ganciclovir. The T-cells are then introduced into the patient alongside the haematopoietic stem cell transplant, and if graft-host disease occurs the suicide gene can be activated, thus getting rid of the T-cells that are involved with the side-effect. The hope is that this will improve the ability of the stem cell transplant to replace the leukaemic cells, and reduce the risk of graft-host disease.
Chronic myeloid leukaemia differs from AML in that its development is slower, and children are much more likely to be affected. Once it has progressed, the overall survival rate is poor. It is a little less common than the acute form of the disease, with 1.2 in 10,000 people in the EU being affected.
One potential product in development for this disease, a different cell-based treatment, is from a joint venture between Gamida Cell and Teva. The product, StemEx, is in Phase III for both CML and AML, and is made from ex vivo expanded umbilical cord blood cells. If no suitable donor can be found, then a bone marrow transplant is unlikely to succeed, and this product is designed to offer an option for patients for whom there is no matched donor.
The stem cells are taken from donated umbilical cords, but because only tiny numbers are present in the cord, they are cultivated using ex vivo expansion to increase the number of cells. To do this, the level of free copper in the cells is modified, which allows the cells to self-renew outside the body on a large scale. They are transplanted alongside non-expanded cells from the same cord.
Acute lymphoblastic leukaemia (ALL) is a cancer of the lymphocytes. In patients with this form of the disease, the lymphocytes multiply far too quickly and are short lived and, as a result, there are many immature white blood cells circulating in the blood. These do not function properly, and ultimately overwhelm the bone marrow, replacing both white and red blood cells as well as platelets. It is the most common form of leukaemia in children, and while it can often be treated, there are still some patients for whom existing therapies fail. About one in 10,000 people in the EU is affected.
SymbioTec’s Oncohist, recombinant human histone H1.3 and recombinant human N-bis-met-histone H1.3, works in a completely different way from current ALL therapies. The histone proteins play a crucial role in the correct coiling up of DNA within cells into chromosomes, and while they are normally only present within cells, cancer cells also have them on the surface. The first histone in Oncohist, histone H1.3, is identical to that found in human cells, and the second, the N-bis-met form, is turned into it in the body. The idea is that they will attach to the histones on the surface of the cancer cells, forming large aggregates that create channels in the membrane. Ultimately, the cell walls break, killing the cells.
A very different product, Oncaspar from Enzon, has recently gained approval in ALL. It is a pegylated form of the enzyme L-asparaginase, with the pegylation introduced to reduce the rate of clearance from the body, and to limit its interaction with the immune system. The enzyme itself breaks down the amino acid L-asparagine in the blood. While normal cells make their own L-asparagine, some cancer cells, including leukocytes affected by ALL, cannot and thus need to take it up from the bloodstream if they are to grow. The pegylated enzyme mops up the asparagine present in the blood, leaving none behind for the cancerous ALL cells, causing them to die.
A monoclonal antibody for ALL treatment is being developed by German company Micromet, in collaboration with MedImmune. Blinatumomab is a novel form of mAB, a bi-specific T cell engager or BiTE, that targets the CD19 antigen that is present on the surface of malignant B lymphocytes. It also attaches to the CD3 binding site on T cells. As a result, the two cells are linked together, and the T cell is activated. The result is that the T cell is stimulated to kill the attached cancerous B cell. The company is currently enrolling patients in a Phase III trial of the antibody in patients with ALL following promising Phase II results.
The chronic form of lymphocytic leukaemia (CLL) is more common, affecting about 3.5 in 10,000 people in the EU, particularly older people – it is rare in those under 40. Another German company, Immunomedics, is developing a monoclonal antibody, veltuzumab, to treat CLL. The humanised monoclonal antibody attaches to CD20 antigens on the surface of B lymphocytes, killing the cells; it is in Phase II trials.
Another antibody with this target is being developed for CLL – LFB Biotechnologies’ recombinant chimeric MAb against CD20, LFB-R603. The company claims that its specific glycosylation profile gives it powerful antibody-dependent cell-mediated cytotoxicity activity against tumour cells bearing this antigen, an activity that has been confirmed in cellular assays, and the antibody is now in Phase I/II trials.
An antibody product to treat CLL that was recently approved by the FDA is GSK’s ofatumumab (Arzerra), a human MAb for the CD20 antigen and the first drug with this mechanism of action to be approved for CLL. Originally created by Genmab, it is thought to inhibit early stage B-cell activation. It has FDA approval to treat CLL that is refractory to fludaribine and alemtuzumab, as well as conditional approval in Europe. It is also being investigated in follicular non-Hodgkin lymphoma and diffuse large B-cell lymphoma.
Hairy cell leukaemia is an even rarer form of the disease, with fewer than 1 in 10,000 people in Europe affected. Again, too many B-lymphocytes are produced, but in this case there are hair-like projections on the surface of the lymphocytes. Only about 2% of all leukaemias are of this type, and long-term survival rates for patients are poor.
MedImmune’s moxetumomab pasudotox, or CAT-8015, is being developed for several forms of leukaemia, including hairy cell; it also has potential in CLL, ALL in children, and non-Hodgkin lymphoma. The drug is an immunotoxin made up of the binding portion of a murine anti-CD22 antibody fused to a truncated form of the Pseudomonas exotoxin, PE38. CD22 is expressed in most B cell malignancies. The antibody binds selectively to the CD22 antigen, is internalised and processed, and this releases the cytotoxic PE38 part of the drug, which inhibits protein translation and leads to cell death.
Another form of blood cancer, multiple myeloma, affects about two in 10,000 Europeans. It is a cancer of the plasma cells, which are white blood cells that produce antibodies. Again, the abnormal cells build up in the bone marrow, with the uncontrolled reproduction of these cells causing the bone marrow to fill up with immature, abnormal plasma cells. The result is, once more, interference with the production of white and red blood cells and platelets, causing problems such as anaemia, bone pain, fractures and kidney disease.
Several drugs are already licensed to treat multiple myeloma in the EU, but current treatments such as chemotherapy, steroids, thalidomide and bone marrow transplants frequently prove inadequate, and the long-term prognosis is poor.
Several investigational products have been given orphan designation. One of these, Immunomedics’ milatuzumab, is a humanised monoclonal antibody against the CD74 antigen, the first with this target; it is also being investigated in CLL and non-Hodgkin lymphoma. The CD74 antigen is found in several forms of cancer, both blood and solid tumours, but its expression in normal tissues is limited. As the antigen internalises rapidly, it is also a good candidate for a drug conjugate, and milatuzumab conjugated to doxorubicin is now in Phase I/II trials in multiple myeloma.
Another antibody being developed for this indication, BioInvent’s BI-505, binds to the adhesion protein ICAM-1, also known as CD54, which is overexpressed in a variety of tumours, but expression is low in normal tissue. BI-505 is a human anti-intercellular adhesion molecule-1 monoclonal antibody that was identified via a screening programme looking for apoptotic antibodies, and the company says that previously the ability of anti-ICAM-1 to lead to apoptosis had not been recognised. As a result, they hope their antibody will prove active in patients who do not respond to current therapies, and clinical trials are under way.
Centocor (now Centocor Ortho Biotech following its merger with Ortho Biotech Inc) is investigating a chimeric anti-interleukin-6 antibody, CNTO 328. The MAb, derived from both human and mouse proteins, works by blocking the IL-6 receptors, with the aim of reducing inflammation and tumour growth, and Phase I trials have taken place in several forms of cancer, both blood and solid tumours. A Phase II trial in patients with relapsed or refractory multiple myeloma was sufficiently promising to warrant the initiation of Phase III trials.
ImmunoGen’s lorvotuzumab mertansine combines a CD56 monoclonal antibody with a macrolide cytotoxic agent. It is being investigated in several forms of cancer, including relapsed or refractory multiple myeloma, and is in Phase I trials for this indication. Early data from a dose escalation study have already shown that it has an effect as a single agent in heavily pretreated patients, and further data are expected to be announced in the next few months.
A related investigational drug from Biotest in Germany combines maytansine with an anti-CD138 monoclonal antibody. This antigen helps the B cells stick together and also to the extracellular matrix as they enter the bone marrow, and the antitubulin drug to which it is conjugated is designed to prevent cell replication and thus lead to cancer cell death.
lymphocytic leukaemias
Lymphomas are cancers of the lymphatic cells, and generally involve some form of solid tumour within the lymphoid cells. There are many different forms, some of which are relatively treatable, but some other rarer forms pose much more of a therapeutic challenge. They are related to lymphocytic leukaemias which, unlike lymphomas, rarely form solid tumours.
First identified by Thomas Hodgkin in the 1830s, Hodgkin lymphoma affects about 1 in 10,000 people in the EU. It occurs in both adults and children, and while it can usually be cured if it is diagnosed and treated early, in some patients it is life-threatening.
Seattle Genetics’ brentuximab vendotin consists of a monoclonal antibody against CD30 joined to the cytotoxic agent mono-methylauristatin E, which is taken up by the cells after the antibody attaches to the CD30 antigens on the surface of the B-cells and is internalised. Phase III trials in Hodgkin lymphoma patients having undergone bone marrow transplants have already taken place, and the company submitted a biologics licence application to the FDA in February for use in relapsed or refractory Hodgkin lymphoma; its partner Millennium intends to apply for marketing authorisation in Europe imminently. This antibody drug conjugate is also in Phase II trials for patients with a different form of lymphoma, anaplastic large cell lymphoma, a cancer of the T-lymphocytes, which affects just one person in 50,000 in Europe.
Seattle Genetics antibody-drug conjugate (ADC) technology empowers monoclonal antibodies targeted to tumour cells by attaching them to potent anticancer agents. The technology employs synthetic, highly potent cell-killing agents called auristatins (such as MMAE and MMAF) and stable linker systems that attach auristatin to the antibody. Seattle Genetics’ novel linker systems are designed to be stable in the bloodstream and release the potent cell-killing agent once inside targeted cancer cells. This approach is intended to spare non-targeted cells and thus reduce the toxic effects of chemotherapy while enhancing the antitumour activity.
German biotech Affimed Therapeutics has developed AFM13 to treat Hodgkin lymphoma. AFM13 is currently being tested in a phase I clinical study. This is a bispecific tetravalent human antibody that targets both CD30 antigens on tumour target cells and macrophages, and CD16a antigens on NK cells as immune effector cells. The idea is that, by binding to both cells simultaneously, the NK cells and macrophages will be directed to the lymphoma cells to kill them. The company also believes it will be effective in anaplastic large cell lymphoma (see Figure 1).
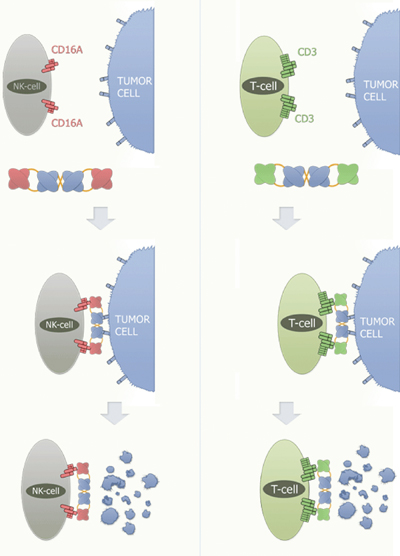
Figure 1: Mode of Action of Affimed TandAbs recruiting NK-cells/T-cells
Illustration courtesy of Affimed
The TandAb crosslinks the NK-cell or T-cell with the tumour cell. This cross-linking induces antibody-dependent cell cytotoxicity (ADCC) and the subsequent destruction of the tumour cell. Affimed´s RECRUIT-TandAb is able to activate immune effector cells such as Natural Killer Cells (NK-cells) and T-cells.
For the recruitment of NK-cells, Affimed has developed a highly specific and potent antibody against the FcyRIIIa (CD16A) receptor, and for the recruitment of T-cells, an antibody with high affinity against the CD3 receptor. The RECRUIT-TandAb binds to a target cell molecule, e.g. CD30, with two of its binding sites, and to the CD16A receptor, with the other two binding sites. This crosslinking event initiates the killing activity of the respective NK-cell through ADCC.
Granules containing cell lysing components, such as perforin, granzyme and lysosomal enzymes, are transported towards the cell membrane of the NK-cell and subsequently secreted into the extracellular matrix. Perforin causes the formation of pores in the target cell, thereby facilitating the entry of the cell lysing components. A similar mode of action occurs when using T-cells as immune effector cells. CD3 is part of the T-cell receptor complex.
If the TandAb binds with its anti-CD3 binding sites to T-cells while simultaneously binding to a molecule on the surface of a tumour cell, the T-cells are activated to induce cell lysis of the targeted tumour cell (ADCC).
Another form of lymphoma, mantle cell lymphoma, is the target of a cancer vaccine from Biovest. This is a rare form of non-Hodgkin lymphoma, affecting fewer than 0.3 of 10,000 people in Europe, and representing about 6% of all NHL cases. It is difficult to treat and rarely cured, with a median survival of 3–6 years.
The vaccine, BiovaxID, is prepared by extracting a protein from cancer cells taken from the patients’ own lymph nodes. This is attached to keyhole limpet haemocyanin, a protein from sea snails, which enhances the body’s response to the vaccine.
The aim is to activate the immune system by priming it with cancer cell proteins, resulting in the cancer cells themselves being attacked. It also has potential in other B cell blood cancers, such as follicular lymphoma.
Another cancer vaccine, IdioVax, is being developed by CellGenix to treat diffuse B cell lymphoma. The most common form of cancer of the lymphatic system, it affects about 2 in 10,000 Europeans, and is characterised by B-cells multiplying too quickly and living for too long, leaving too many of them in the lymph nodes; the first symptom is a lump in the neck, armpit or groin resulting from an enlarged lymph node. Again to make the vaccine, cancer cells are taken from the patient, proteins specific to that patient’s cancer isolated, and combined with other immune-priming components before being injected back into the patient to stimulate an immune response against the cancer cells.
