In this second article exploring new methods of quantifying and analysing the performance characteristics of controlled release products,* Jo Smewing, applications manager, Stable Micro Systems, outlines tests for parenteral forms of controlled delivery systems
Developments in transdermal drug delivery methods are paving the way to the future of systemic drug administration. As skin is both the largest and most accessible organ in a human body, pharmaceutical manufacturers are keen to take advantage of it as a drug delivery platform. The transdermal route helps to reduce the risks and costs of intravenous therapy that can be both traumatic and intrusive.
Transdermal controlled release (CR) systems usually combine a therapeutic component with an adhesive formulation that ensures a continuous delivery of the active ingredient through unbroken skin at a constant absorption rate. Skin adhesion and drug compatibility determine the efficacy of transdermal applications. Adhesive properties are critical because the amount of medication delivered is directly proportional to the skin contact area.
Transdermal systems need to cause minimum skin disruption, be easy to handle and be equally effective for the infinite variations in human skin. In vitro tests are commonly used to determine and compare tack, peel adhesion and shear characteristics of transdermal samples. Automated texture analysis, facilitated through the use of a TA.XTplus texture analyser, for instance, allows such testing to be carried out in objectively controlled conditions that closely simulate real applications.
Creams, lotions and topical gels are typical examples of dermal delivery drugs. Their retention on the affected area is challenged by various shearing forces such as breathing, swallowing and flexing of the skin. Their performance depends on viscosity, spreadability, bioadhesion and good drug release and absorption. Texture Profile Analysis (TPA) allows manufacturers to assess and fine-tune the mechanical characteristics of these products, optimising their acceptability and efficacy. TPA is a rapid and simple technique for measuring the hardness, adhesiveness and compressibility of semi-solid or self-supporting substances
(Fig. 1). A large cylindrical probe compresses a sample, held in a container whose circumference slightly exceeds the circumference of the probe. Equally effective is a back extrusion test (Fig. 2) whereby a disc is pushed into a viscous sample to measure the resistance to flow upon insertion and then resistance to withdrawal.

Figure 3: Peltier plate
patch adhesion
Adhesive patches are widely used in highly targeted and custom-designed applications. A simple test to measure the adhesive properties of a transdermal patch involves a domical or spherical probe descending onto a sample, which has been attached to the platform with double-sided adhesive tape. After allowing adhesive bonds to be created for a defined time, the probe withdraws. The strength of adhesion, indicated by the force required to detach the probe from the sample, is recorded by the texture analyser.
Certain elements must be controlled to ensure maximum repeatability and consistency of results. These include the probe’s travelling speed, the amount and duration of applied force and withdrawal distance and speed. Ideally, a Peltier Plate (Fig. 3) should be used as a controlled testing platform to prevent temperature fluctuations from affecting results. The data obtained from adhesion tests are then transformed into a graph that helps to analyse the results and optimise the characteristics responsible for the product’s performance (Graph 1).
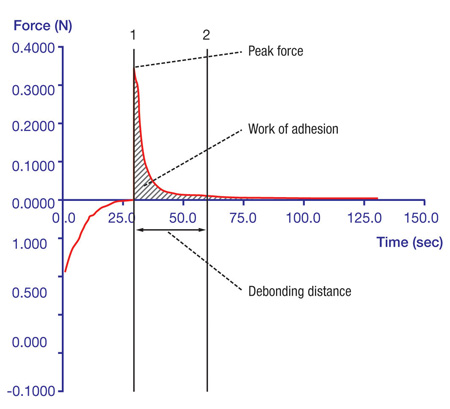
Graph 1: Results of adhesive tests
The efficacy of topical films is dependent on correct application to the skin and the retention of the product on the body for a sustained period. Incorrect application can result in inconsistent results from the same medication, so new moisture-activated patch applications have been developed to overcome these issues. In contrast to topical creams, the fixed dimensions of transdermal patches ensure better coverage of the affected area and control the release of active ingredients as well as their retention.
In a simple test to measure the bioadhesion of topical films, an adhesive sample is attached to a cylinder probe, which is then lowered onto a neonate porcine skin sample (which closely resembles the human epidermis). The texture analyser then measures the force required to remove the probe from the skin surface.

Figure 4: Adhesive indexing system with a flexible substrate clamp
To improve the test conditions to cater better for the requirements of hydrogel films, a Flexible Substrate Clamp can be used to secure both the sample and the skin in place. The device consists of a multi-slot plate and a clamp fixture and works in conjunction with an Adhesive Indexing System (Fig. 4). The skin sample is held under the plate and the upper fixture lowers the sample down into each slot. The texture analyser then records the force required to withdraw after adhesive bonds have been formed.
peel profiles
To assess the peel strength of a typical bi-laminar patch formulation, a cyanoacrylate adhesive is used to attach wetted neonate porcine skin to the sliding platform of a 90 Degree Peel Rig (Fig. 5). A sample of the patch is secured vertically; held at the top with a clamp, and at the bottom attached to the skin. As the clamp moves upwards, the sliding section of the rig moves horizontally, forming a 90° angle between the interface of the skin sample and the adhesive patch. A texture analyser records the force required to peel the patch off the skin sample. A 180° peel test improves accuracy by maintaining a constant force before the clamp withdraws up with an automatic roller.

Figure 5: 90 Degree Peel Rig assesses peel strength
Mucosal delivery requires a drug delivery system to be attached to a target area of mucous membrane, such as a part of the gut, for a predefined length of time. Its success depends on the strength of the product’s mucoadhesive bonds, which can be measured using the TA.XTplus.
Simple adhesive tests, similar to those described above, provide details of the mucoadhesive properties of films, powders, gastrospheres or polymers. But a more recent development, the Mucoadhesion Rig, closely replicates in vivo conditions with a porcine membrane sample positioned in a fixed volume of temperature-regulated gastric fluid. A cylinder probe, with a sample attached to it, descends into the vessel until the sample is in contact with the tissue (Fig. 7). The texture analyser controls the force and time of contact between the two surfaces and records the force that separates the surfaces, thereby measuring the mucoadhesion strength.

Figure 6: 180 Degree Peel Rig offers greater accuracy
A Gel Mucoadhesion probe was recently developed to enable the successful attachment of a hydrogel sample to the underside of a probe, in order to perform a mucoadhesion test. The new probe incorporates an inverted cone shape with machined concentric grooves on its surface. These enable consistent volumes of hydrogel substances to set upon and remain attached to the probe surface for the duration of the test.
Mucoadhesive polymers allow formulations to be attached to mucosal surfaces and deliver active ingredients to required sites, offering such advantages as good retention and easy application. Syringeability and viscosity are the governing characteristics of such formulations and can be assessed in a compression test, where a texture analyser expels equal amounts of samples from identical plastic syringes. The samples’ syringeability is calculated from the resistance to the expression force. Typical results can be seen in Graph 2.

Figure 7: The Mucoadhesion Rig
parenteral delivery
In recent years, implants and injectable polymers have revolutionised parenteral delivery, but the ratio of inactive to active ingredients must be optimised to ensure both a therapeutic effect and ease of use.
Gel substances and hydrogel polymers are increasingly prominent in mucosal and other delivery systems. The accurate definition of gel properties is pivotal for manufacturers of products as diverse as wound dressings, jelly lubricants and contact lenses.
Aqueous gel is tested for its resistance to stress using a cylinder probe that is lowered onto the sample at a fixed speed and distance until the gel surface is fractured. The peak force required to fracture the sample indicates gel rupture strength. In the case of self-supporting gels, manufacturers may wish to look into the product’s elastic moduli.
Elastic moduli give an indication of a substance's tendency to be deformed elastically when a force is applied to it. This can be done by lowering a cylindrical probe onto a gel sample placed on sandpaper to prevent slipping. This test allows manufacturers to evaluate gel strength, elasticity and gel rupture force.
Similar to the abovementioned mucoadhesive polymer syringeability test, the Syringe Test Rig measures the injectability of CR gels and polymers. The test determines the pressure force required to move 8cm of cement through a 3mm diameter polyethelyne catheter. The Adhesive Indexing System can also be used to measure the setting times of varying cement formulations.
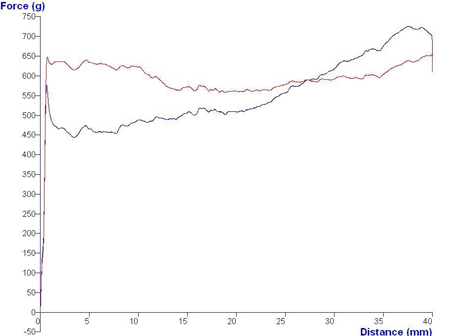
Graph 2: Results of syringe tests
ocular delivery
As with dermal tissues and oral cavities, the treatment of optic diseases also benefits from direct administration onto the affected area. Ocular drug delivery is significantly hindered by poor bioavailability of drugs as the eye is protected by complex defence mechanisms.
Contact lenses improve bioavailability by facilitating prolonged contact with the eye. A rigid lens’s mechanical strength can be measured by clamping a cross-section of a dumbbell-shaped sample and measuring the maximum force required to stretch it to breaking point. The test gives indication of the material’s suitability for pharmaceutical applications.
Soft contact lenses, however, differ as they have a high hydrogel content that has a very low modulus of elasticity. Their properties are therefore better tested in a way similar to the gel elasticity and rupture force test described above.
inhalation
An addition to the variety of controlled dosage forms, inhalation drug delivery via metered-dose inhalers (MDIs) allows the administration of medication straight into the patient’s lungs. This route avoids the delay of digestion, delivering the medication to the affected area with speed and precision. As inhaler users may suffer from weakness when the drug dose is needed, it is important to monitor the ergonomic functionality of inhalers.
The efficacy of MDIs is determined by the performance of the metering valve, which releases the drug from the container to the area of administration. For this, the Inhaler Support Rig vertically secures an inhaler while a hemispherical probe closely imitates the finger action that releases the drug. The texture analyser measures the force required to press the inhaler and expel the medication.
In conclusion, to release active ingredients gradually and predictably, CR drugs must adhere reliably to the target organ and withstand wear and tear without compromising their functionality. Testing in conditions that closely resemble reality enables manufacturers to optimise performance of their products. Stable Micro Systems offers a variety of quality control systems that enable it to evaluate precisely the physical properties of diverse drug forms.
Accurate quality control tests allow pharmaceutical manufacturers to develop formulations that combine optimal therapeutic efficacy with vital user-friendliness: less frequent administration, improved convenience and greater patient compliance. And ultimately, these two qualities are pre-requisites for long-term success.
*The first article in the series was published in Manufacturing Chemist, Feb 2010, 81, 3, 40-41. It can be also found at www.manufacturingchemist.com/technical/article_page/Discover_the_physical_limits/43849
