Several emerging and significant healthcare trends are forcing the drug industry to reconsider its strategies. An aging population adds pressure to deliver improved healthcare at reduced costs, while demographic shifts in purchasing power create new markets. In parallel,there are a number of exciting proteins that the industry is struggling to develop as products. To put it simply, there are many challenges to meet.
Of course there are many ways in which the healthcare sector might and will create affordable and accessible healthcare via innovations such as predictive biomarkers, "personalised" medicine, biosimilars, superiors, betters, etc. One certainty is that every protein product has to be delivered to the patient at the right dose to the right site.
Parenteral delivery of therapeutic proteins is often affected by low stability, immunogenicity, or lack of efficacy due to short protein half-life or poor bioavailability; these are issues for numerous peptides, whether approved or in development. It often leads to both high and frequent dosing, along with high peaks or low levels of a biopharmaceutical that can give unwanted side-effects or limit therapeutic benefit. Elevated patient dosing means an extra burden on manufacturing capacity and treatment costs. Indeed, developing protein therapeutics is often challenged by poor and complex manufacturing. So any delivery technology needs to lower not only the protein production costs but also the associated medical and administration costs.
Biologics have a growing presence in the therapeutics market. Novozymes Biopharma has positioned itself to support the needs of this industry by providing processing solutions for biologics. Novozymes is not a pharmaceutical company but works with partners throughout the entire development pathway for a particular therapeutic molecule, offering integrated expertise in the regulatory process, protein expression, process development and bioprocess manufacturing required to deliver a commercial-scale bioactive in a robust, reproducible and cost-effective manner, helping customers expedite their product lifecycle.
It is worth considering the analogy of proteins with the vulnerability of man when in space - an astronaut can survive for a finite time performing limited activities; the duration and extent of those activities are extended when attached to a spaceship. One can envisage a target protein in the body as an astronaut struggling to stay in space for a long time, but if that target protein is somehow attached to albumin (a molecule that has a naturally long residence time in man) like a "mothership", then the target protein can stay in the body long enough to be an effective medicine.
So how can Novozymes claim that using albumin is innovative when it has been known for many years? Because it has taken a novel and inventive approach by using albumin's benign properties with its exceptionally long in vivo half-life and applied genetic engineering to create therapeutic albumin fusion molecules. Albufuse is a proprietary albumin fusion platform technology for producing albumin joined to a therapeutic protein or peptide, defined at the genetic level (see Fig. 2). The recombinant DNA is engineered typically into a host Saccharomyces cerevisiae yeast cell. Albumin fusions have been shown to improve the pharmacokinetics of the protein.
The promising field of biologics is constrained by a number of factors, in particular issues such as low stability and lack of efficacy, due to poor half-life and bioavailability. These issues can result in high peaks or low levels of the drug in the patient, resulting in unwanted side-effects and/or limit the therapeutic benefit (see Fig. 4). Developing protein therapeutics is also challenged by delivery and complex manufacturing. Novozymes" albufuse and associated technologies have been proven to manage these difficulties.
medical benefits
A key clinical advantage of albufuse is an increase of in vivo half-life of the therapeutic protein fused to the albumin. This confers advantages for patients, such as significantly reducing the frequency of administration of the therapeutic protein, thereby reducing the overall dose. Since some biopharmaceuticals have to be administered by a nurse at home or at a clinic, the number of visits can be dramatically reduced, resulting in better compliance and ease of use. Additionally there is improved stability and shelf-life of the proteins.
Albumin is a natural, abundant carrier molecule, present in high volumes in the bloodstream and has a naturally long half-life of 20 days. It has no endogenous activity and is ideal as an effective carrier molecule for transporting various molecules around the body. This makes albumin a natural choice as a drug delivery vehicle.
The albufuse concept from Novozymes Biopharma is suitable for many peptides and proteins. There are several binding points on the heart-shaped albumin molecule where proteins and peptides can be placed (see Fig. 1). It is even possible to fuse two different proteins together with albumin in the same recombinant molecule to give two different functions at the same time. The new technology is therefore not just suitable for improving existing products but also for making novel products, such as bi-functional proteins.
Albumin fusions offer new opportunities for the development of new drugs and treatments, creating value by providing solutions to the pharmaceutical industry. Albufuse can achieve significant cost savings because of the ease of manufacture of the protein, with none of the additional post-manufacture purification steps required by some technologies, such as conjugation by PEGylation: it is a natural and simpler alternative to chemical conjugation.
A significantly reduced dose means that the treatment is more cost-effective, which is becoming crucially important with the increasing focus on healthcare costs and accessibility to medicine. There is also a reduced risk of side-effects as a lower dose rate means that the toxicity level of the protein may not be reached. Instead the drug dose remains within the therapeutic range, increasing the patient's tolerance to the drug. Furthermore, it has been demonstrated that albufuse products have a more favourable tissue distribution within the body, reducing the risk of localised retention at the site of administration.
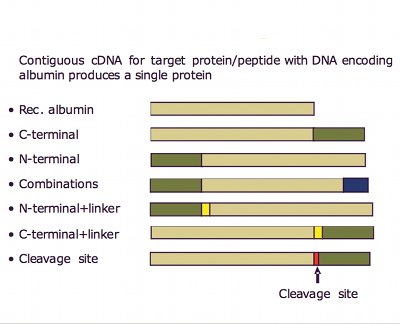
Fig. 3: Albumin fusions have been shown to improve the pharmacokinetics of the protein
example drugs
Interferon is used to treat people suffering from chronic hepatitis C viral infections; an estimated 170 million people are infected (3% of the population), growing at more than three per year. However, with a normal halflife of ~5 hours, interferon is soon lost from the body. One way to make it last longer is to use PEGylation, whereby a chemical is bolted on to the interferon. This only extends the halflife to 35 hours. Alternatively, by fusing interferon to human albumin, the half-life is increased to one week.
Albufuse has been licensed by Human Genome Sciences (HGS) in collaboration with Novartis to make a drug called Zalbin - a genetic combination of albumin and interferon. It has successfully completed phase 3 clinical trials and is now with the various authorities for marketing approval, with an expectation that it will be launched on the market by late 2010. Zalbin requires half the number of injections compared with PEGylated interferons and phase 3 results demonstrated that Zalbin offers at least comparable efficacy, comparable safety and the potential for less impairment of health-related quality of life.
Zalbin is just one of the drugs under development featuring albufuse technology. Other companies, such as Teva, are also using the technology under licence. One of Teva's albufuse molecules in clinical development is Neugranin - albumin genetically linked to GCS-F for chemotherapy-induced side effects (Febrile Neutropenia Associated with
chemotherapy).
CSL Behring has also employed this technology in developing its next-generation plasma protein biotherapeutics. The proof of principle study showed for the first time that it was possible to fuse the coagulant factor VIIa genetically to human albumin, thereby meeting an unmet need in haematology by improving pharmacokinetic parameters.1 CSL has also shown equivalent benefits for haemophilia B patients using Factor IX fused to albumin.2
The yeast Saccharomyces cerevisiae has a long and successful track record in the production of protein therapeutics, such as insulin. Based on a proprietary plasmid and strains, Novozymes" versatile system allows the expression of an extensive range of proteins, representing many classes of biotherapeutics from antibody fragments and protease inhibitors to anti-inflammatory polypeptides, growth hormones and albumin fusions. A series of strains has been developed with all the desirable attributes essential for use in manufacturing.
Novozymes Biopharma has proven the suitability of its strains at cGMP manufacturing scale through its lead product, Recombumin, the only commercially available recombinant human albumin authorised for use in human therapeutics. Importantly in this context, Novozymes'expression and manufacturing technologies are used in the production of albufuse molecules including Zalbin and Neugranin.
Albufuse technology offers clinical advantages to the patient and reduces the cost of albumin-fused drug candidates. It has also been shown to improve bioavailability and increase in vivo half-life from "minutes to hours, hours to days," resulting in less frequent administration, reduced side-effects and improved patient quality of life.
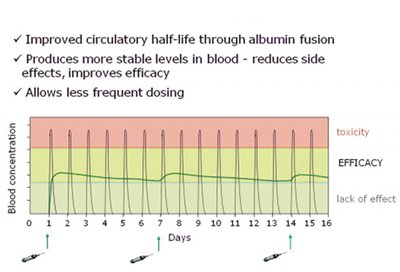
Fig. 4: Schematic showing how albufuse avoids high peaks or low levels of drug occurring following delivery to the patient
In addition, albufuse technology offers a single-step expression solution as an alternative to lengthy and costly post-production chemical processing required of other methods for extending circulatory half-life and is potentially a more consistent and natural alternative for effective protein drug half-life extension.
The low production costs achieved through high expression levels in the S. cerevisiae expression system further emphasise the breadth and significance of the potential advantages albufuse technology offers for future biopharmaceutical product enhancement and development.
references
1. Schulte, S. (2008), Thrombosis Research, Vol.
122
2. Metzner, HJ. et al. (2009), Journal of
Thrombosis and Haemostasis, Vol. 7,
Supplement 2
CPhI Innovation Awards
This inspiring solution to improving the efficacy of therapeutic proteins won Novozymes Biopharma a Bronze Innovation Award at CPhI 2009 in Madrid. Dave Mead (below) was presented the award on behalf of Novozymes Biopharma during the show. Gold and Silver winning innovations were featured in the January and February issues of Manufacturing Chemist respectively.
