The analytical testing of cell cultures used in monoclonal antibody (mAb) production calls for rapid, robust antibody titre measurements with a wide dynamic range to monitor product yields throughout a bioreactor run.
Generally considered to be the benchmark for characterising upstream bioprocessing efficiency, titre measurements indicate the proportion of desired product relative to nutrients and by-products for a given bioreactor volume. As well as a wide dynamic range and robustness, accuracy, speed and specificity are critical parameters in titre measurements.
In a production environment, the turnaround time for protein titre from an offline quality control (QC) laboratory can be too long for the titre to be an actionable data point. The biotherapeutics industry would benefit from a fast, reproducible and automated platform that can provide protein titre information in real-time to extract maximum value from a bioreactor run.
High performance liquid chromatography (HPLC) with Protein A affinity capture is one of the most widely used methods for titre measurement and is considered to be the “gold standard.” Although a rapid and highly specific technique in itself, HPLC capability is rarely accessible at-line, making measurements at multiple stages of the bioprocess challenging.
Titre measurements are important from early cell line development through to manufacturing, so alternative, automated methods are increasingly being considered as a replacement for HPLC.
Process and analytical development
Successful process analytical technology (PAT) informs the design, analysis and control of manufacturing processes. PAT focuses on monitoring the critical process parameters (CPPs) affecting the critical quality attributes (CQAs) of biopharmaceuticals in real-time, and a number of techniques have been developed to optimise cell attributes and culture media components, for example.
Abnormal product titre may be the first sign that the bioreactor is compromised, so online and at-line sampling for rapid titre determination can ensure efficient biotherapeutic production. The measurement of antibody titres is therefore a CPP for optimising cell culture conditions.
Process development and upstream bioprocess optimisation emphasise the robust generation of high product titre, high productivity and defined quality.
The US Food and Drug Administration (FDA) recommends that manufacturers use PAT to develop and implement effective and efficient innovative drug development, manufacturing and quality assurance, and the International Conference on Harmonization (ICH) has included the concept of PAT and quality by design (QbD) in its Q8–11 guidelines.1–5
Successful PAT relies on the analysis and monitoring of CQAs within the bioprocess, such as monitoring IgG titre, and high quality data and real-time access can help to achieve this. There is also a growing need for QbD as a regulatory requirement for process and analytical development, and its implementation could lead to more robust and efficient mAb production.6
Evaluating a novel protein analyser
A recent study compared a novel chromatographic protein analyser (Tridex, IDEX Health & Science) with HPLC for titre measurements from spiked bioreactors.7 The protein analyser uses a trap-and-elute technology to obtain automated at-line and online measurements of antibody titres from stirred bioreactors. It quantifies the amount of IgG in a cell-free sample, providing rapid and accurate results with an easy-to-use interface, requiring minimal training.
Assay linearity and accuracy were evaluated for a measurement range of 0.1–6.5 g/L using a bioreactor spiked with bovine serum albumin (BSA) and human IgG. Calibration curves were generated using a human IgG isotype control standard (Figure 1) and showed excellent fit (R2) of linear regression across the calibration range.
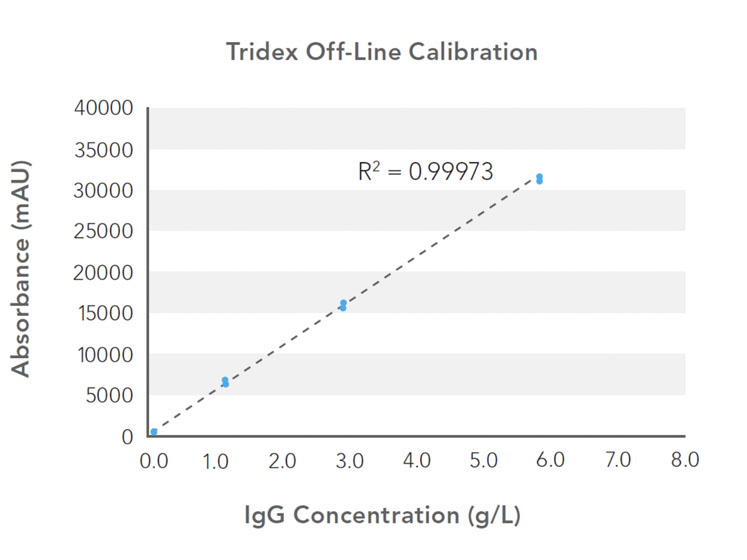
Figure 1: Calibration curves generated using a human IgG isotype control standard: standard measurements at 0.1, 1.0, 2.5 and 5.0 g/L were used to generate protein analyser and HPLC calibration curves
The extended dynamic range allows extrapolation to higher titre values, which is particularly useful given that many bioprocessing systems are now producing up to 10 g/L in fed batch processes.8–10
The correlation of the protein analyser and HPLC titre measurements is shown in Figure 2, with excellent agreement across the titre range. The measurement results were unaffected by changing BSA levels up to 2.5% (v/v) and demonstrate the successful application of an automated at-line protein analyser for titre measurement.
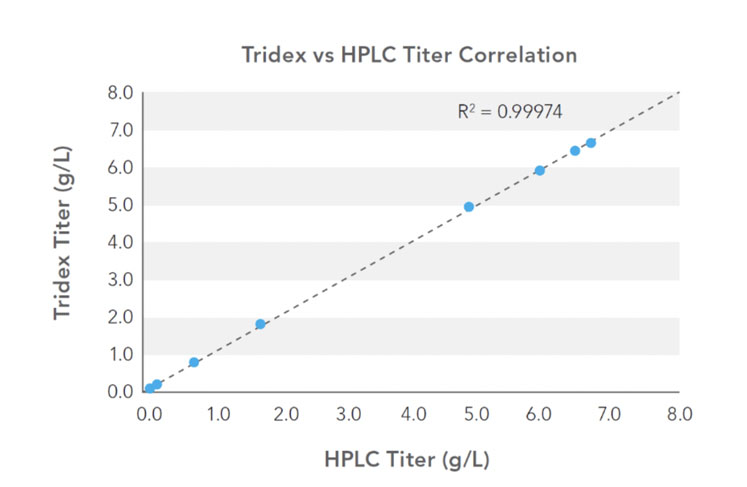
Figure 2: Correlation of protein analyser (Tridex, IDEX Health & Science) and HPLC titre measurements
Titre measurements were obtained in less than five minutes for offline measurements with the protein analyser, and less than 10 minutes for automated at-line measurements. Excellent intra-assay precision (CV <3%) was obtained for both offline and at-line measurements using the protein analyser, and a linear response of the analyser was demonstrated from 0.1–6.5 g/L without calibration or sample dilution.
In a separate study, the protein analyser was also compared with HPLC and biolayer interferometry (BLI) — an optical quantitation technique. In this study, a separate online sampling system was programmed to automatically withdraw a user-defined volume of cell-free sample from the bioreactor containing a stable CHO cell line expressing a humanised IgG antibody.11
Sample was then delivered to the protein analyser system in real-time at six-hour intervals for 19 days. The antibody titre of the cell-free sample was measured by the protein analyser and compared with offline measurements using BLI and HPLC (Figure 3).
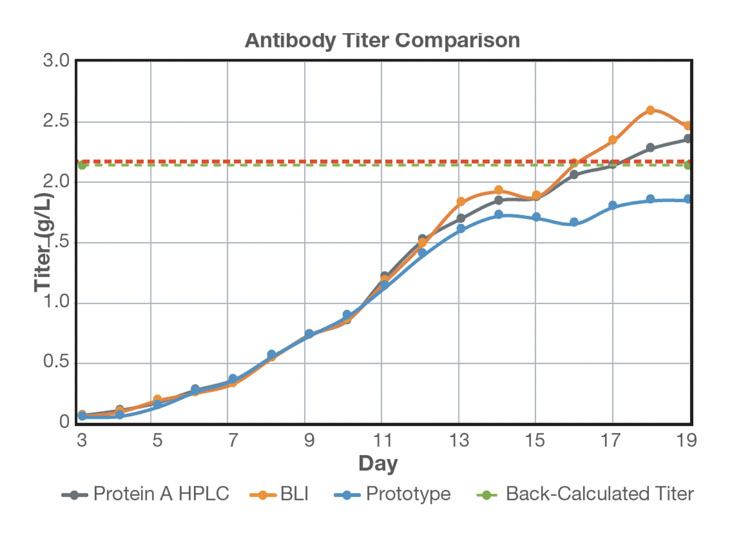
Figure 3: Comparison of real-time antibody titre with offline antibody titre measurements: an average of three measurements per day was plotted for offline HPLC and BLI analysis; protein titre at harvest was back-calculated following Protein A purification
Titre values up to 10 g/L were measured without dilution using the protein analyser and the results show that the range, accuracy and robustness of the chromatographic system for at-line measurement of mAb titre were comparable with offline measurements using BLI and HPLC.
Real-time measurements with the protein analyser could potentially enable process controls based on titre. The analyser streamlines the method development and eliminates the trial and error associated with BLI and other analyses used today. It enables single titre measurements on demand without requiring economies of scale.
Continuing bioprocess optimisation
The correlation between titre measurements from the protein analyser, HPLC and BLI methods highlight the practical utility of the analyser in biopharmaceutical manufacturing.
Although, currently, the most common approach to biopharmaceutical manufacturing is fed batch suspension culture, continuous production (perfusion) is increasingly the goal because of its improved scalability, product quality, stability and associated cost savings. This trend also aligns with the regulatory move towards PAT and a QbD approach.
Despite this emphasis from regulators, the uptake of PAT has not been as widespread as QbD, potentially owing to the complexity of the bioprocess. Against this background, the ability of the protein analyser to directly measure the titre of any antibody produced during a fed batch or perfusion bioreactor run has the potential to close this gap.
Real-time titre measurements can provide valuable insights for cell line development and process development optimisation, and the protein analyser could become an invaluable tool for measuring CPPs in real-time during the manufacture of an antibody for therapeutic use.
References
- US Food and Drug Administration, Guidance for Industry PAT – A Framework for Innovative Pharmaceutical Development, Manufacturing, and Quality Assurance (2004): www.fda.gov/media/71012/download.
- www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q8_R1/Step4/Q8_R2_Guideline.pdf.
- www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf.
- www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q10/Step4/Q10_Guideline.pdf.
- www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q11/Q11_Step_4.pdf.
- P. Gronemeyer, R. Ditz and J. Strube, “Trends in Upstream and Downstream Process Development for Antibody Manufacturing,” Bioengineering 1, 188–212 (2014).
- J. Bravo, C. Yuan and C. Love, “Automating At-Line Measurement with a Novel Fit-For-Purpose Chromatographic Device,” poster presentation, IDEX Health & Science, Automated Engineering Services (2019).
- F. Li, et al., “Cell Culture Processes for Monoclonal Antibody Production,” Pharm. Sci. Encycl. 2, 466–479 (2010).
- B. Kelley, "Very Large-Scale Monoclonal Antibody Purification: The Case for Conventional Unit Operations,” Biotechnol. Progr. 23, 995–1008 (2007).
- J.H.G. Chon and G. Zarbis-Papastoitsis, “Advances in the Production and Downstream Processing of Antibodies,” New Biotechnol. 28, 458–463 (2011).
- I. Kottayil, et al., “Novel Device Enables Direct Measurement of Antibody Titers Up to 10 g/L in Real Time Without Sample Manipulations,” poster presentation, IDEX Health & Science (2019).
