A remote audit cannot replace an onsite audit, but in situations where access to a site is not possible, Rephine deems this to be an acceptable solution based on guidance from EU Regulatory Authorities.
Remote auditing allows for detailed examination of documentation including processes and records. The remote audit can act as a bridge between onsite audits and will be sufficient to be used in conjunction with a previous onsite audit report for QP declaration for existing suppliers.
Rephine’s Expertise & Advantage
Rephine’s consultants are probably the most experienced and highly skilled in the industry, and their breadth of knowledge enables them to provide assessments that can be relied upon in these challenging circumstances. In addition to having carried out many physical audits, documentation reviews have been an everyday part of their work, so they are well equipped to provide a realistic evaluation as part of the remote audit.
Rephine has an advantage through its history of auditing and knowledge of sites, some of which may have been audited on more than 4 occasions. This breadth of knowledge at specific sites significantly reduces the risk associated with conducting a remote audit.
Scope
The Rephine remote audit is suitable for any site which Rephine or a Sponsor has audited previously. New suppliers without an audit history are excluded from the scope.
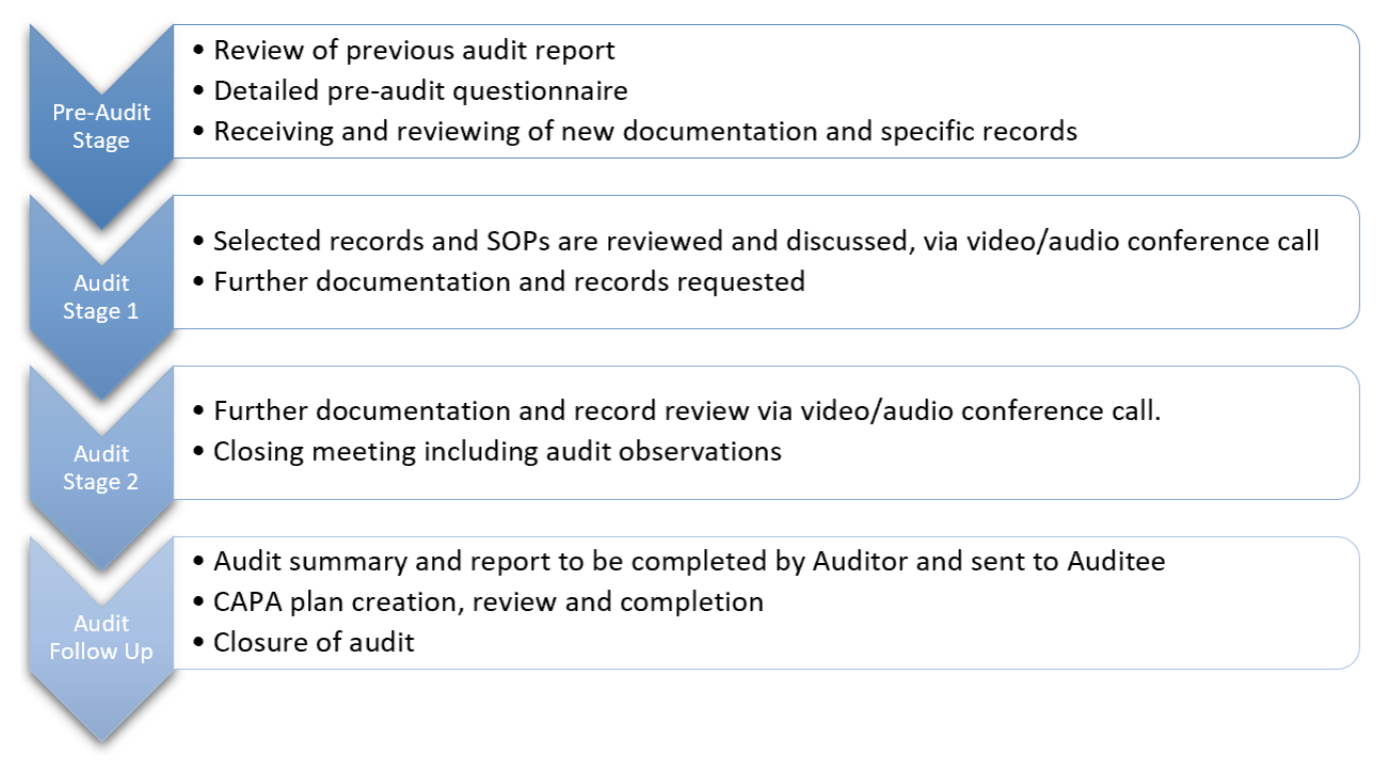
Audit Process
Acceptance for QPs and Regulatory Authorities

QP declaration requires justification if the date of the last audit exceeds a 3-year period and supporting evidence is able to be provided. The Rephine Remote Audit will limit the risk and allow for the continual release of medicinal products.
UK and EU Regulatory Authorities have stated that they will be flexible during this period where access to sites is not possible. Rephine has received positive feedback and acceptance from two major Regulatory Authorities on the concept.
EMA guidance also allows for “paper-based audits”, “when recent positive inspection information is available and where satisfactory audits have been concluded in the past. They cannot replace on-site audits of active-substance suppliers but can be a useful interim and temporary measure within the manufacturer’s audit programme.”
Contacting Rephine
Contact Rephine using enquiries@rephine.com or 0044 1763 853135 to discuss your remote auditing requirements.
