Reaxa ceo, Dr Pete Jackson, illustrates how EnCat technology is proving to be cost-effective in simplifying process work-up for pharma production at scale
Last October, pharma metal extraction technology specialist Reaxa announced additional investment in preparation for commercial-scale production of its multiple-use Nickel EnCat (Ni EnCat) immobilised catalyst. The company’s tenth polymer-encapsulated catalyst, Ni EnCat has been moving through trials at a major pharma player in preparation for bulk scale production of a high-volume API.
Few would argue that catalysis can play an important role in the development of new, greener and cheaper processes for pharmaceutical production, and many companies spend significant amounts of money and time screening to find the optimum catalyst for their application.
The standard approach is:
1. Library screening under standard conditions
2. Selection of lead catalyst based on highest conversion
3. Development of the process conditions to maximise yield
4. Optimisation for scale-up and imple-mentation on plant.
The main problem with the screening methodology is the selection of the initial screen conditions, which may fundamentally discriminate in favour of one set of catalysts over another.
In Example 1, involving a Suzuki coupling using one of Reaxa’s palladium (II) EnCat catalysts, a big yield effect is seen simply by varying the solvent/base combination. It is clear that choosing one of these combinations, as a standard screening condition, could result in this catalyst achieving a range of results anywhere between 8% and 90% yield.
Example 1: Yield variation with Pd(II) EnCat 30 with base and solvent
| Base % | Product after 30 min | Pd in crude product (ppm) |
| Bu4NOH/H2O | ||
| Bu4NOH/MeOH | ||
| Bu4NF | ||
| Bu4NF/ H2O | ||
| (Et4N)2CO3 | ||
| Bu4NOMe/MeOH | ||
However, there can be other disadvantages of this screening approach from the commercial process development perspective. First, these activities may be separated by a considerable time, such that the early research process could be developed many years before scale-up is required. There may well be no prior consideration of the likely eventual site of manufacture or process equipment, or in advance of a formal product release specification being fixed.
In addition, stages 1 and 2 can often be performed by different groups to stages 3 and 4, even within the same company, with differing technical focus – for example, maximising conversion and catalyst efficiency versus optimising isolated yield and plant productivity (cycle times).
In the aforementioned Suzuki coupling example, optimising yield could lead to a higher level of palladium contamination in the product, requiring further reprocessing and purification. From the point of view of the manufacturing chemist or engineer, plant productivity can be a significantly more important driver of process economics than yield optimisation.
This is shown by the two process scenarios in Table 1. Which of these would be considered as the ‘best’ catalyst overall?
Table 1: Two process scenarios
| CATALYST A | |||||
| CATALYST B | |||||
The design philosophy behind EnCat catalysts is to maximise the separation between the metal catalyst species and the reaction products to vastly simplify process work-up – in effect each Reaxa polymer bead acts like a ‘micro-reactor’, making it easy to separate the catalyst from the product at the end of the reaction.
The products obtained from a coupling reaction illustrate this in ‘black and white’ (see Fig. 1). The black product is made using a conventional palladium (Pd) homogeneous catalyst and contains significant Pd contamination, whereas the white product is the same material made using Pd EnCat, requiring no further purification.
It is clear that elimination of multiple work-up and purification stages can lead to the potential for considerable cost-savings and process efficiencies.
While nickel catalysts can be highly effective in pharma processing, there is a major disadvantage, due to residual metal contamination, from dissolved nickel, residual aluminium and, for example, molybdenum additives used to increase the catalytic performance.
Responding to requests from industry, Reaxa has developed Nickel EnCat, an immobilised microparticle nickel (0) catalyst that traps the active catalyst species in a porous polymer bead, preventing product and waste stream contamination.
Furthermore, the Ni EnCat catalyst is safer to use than Raney/sponge-type nickel products, which are pyrophoric. Ni EnCat is categorised as a non-flammable solid, allowing open-air handling not possible with the other nickel catalysts.
process simplification
Sponge-type nickel is used extensively in the production of APIs for the treatment of neuropathic pain. The gamma-aminobutyric acid (GABA) class of compounds is generally obtained from nitrile precursors with the standard formula as shown in Scheme 1, where R1 is usually a carboxylic acid or ester functionality.
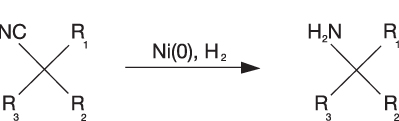
Scheme 1: Synthesis of amine compounds via a Ni(O) catalysed nitrile hydrogenation
Initial studies were performed comparing sponge nickel catalysts with Ni(0) EnCat (with nickel at 10% loading within the polymer bead) in a high-pH aqueous reaction system. In one case, optimisation of the reaction temperature led to a marked improvement in conversion with the EnCat product, allowing a significant reduction in the nickel loading to below 4%, compared with the existing sponge nickel catalyst system, which was used at over 10% loading (see Example 2).
Example 2: Temperature optimisation of a reaction in a high-pH aqueous system using Ni (0) EnCa
| Temp °C | ||||
| 55 | >90% | >90% | ||
| 45 | >90% | >90% | >70% | >50% |
| 35 | >50% | |||
| 25 | <50% | |||
Unlike sponge-type nickel catalysts, the Ni EnCat catalyst does not require high-shear turbine mixing, and filtration times are extremely short due to the EnCat bead size of typically between 100 and 200µm.
Most importantly, analysis by ICP of the metal content of the isolated solid product showed residual metal contamination significantly reduced for the EnCat catalyst (Table 2).
Table 2: Comparison of residual metal contamination
| Aluminium content | ||
| Nickel content | ||
This reduction in impurities gives a significant process benefit compared with results using sponge nickel catalysts, with elimination of purification steps leading to significant yield gains. Additionally, the Ni EnCat catalyst can be reused in subsequent batches, with no loss of activity seen in up to six recycles.
With process optimisation, 45°C was confirmed as the optimum temperature for running this particular reaction, taking into account the process yield, economics, catalyst recycling and operational factors.
Recent work has allowed further development of the Ni EnCat range to include different loadings of nickel encapsulated within the polymer beads – as well as variation of the polymer formulation to change the nano-pores within the polymer, in order to vary reaction kinetics.
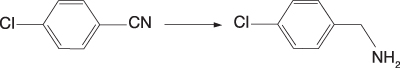
Scheme 2: Standard cholorobenzonitrile reduction
As an example, a range of developmental Ni EnCat products has been used in a standard chlorobenzonitrile reduction (Scheme 2). Increasing the Ni content of the polymer beads leads to the anticipated increase in reactivity, with 25% loaded beads giving complete reaction in under three hours, compared with 8–12 hours for the 10% loaded beads (Example 3).
Example 3: Increasing the Ni content of polymer beads
| Time | 10 | 15 | 20 | 25 |
| 3h | 60% | 90% | 100% | 100% |
| 6h | 90% | 100% | ||
| 12h | 100% | |||
Following the filing of initial patents in mid-2009, Reaxa secured a development grant from NWDA (UK’s North West Regional Development Agency), valued at £146,000, combined with additional financing from investors, to help develop Ni EnCat from lab scale to tonne scale.
The project includes establishing the safety and quality characteristics of Ni EnCat and laboratory trials to formulate use for selected industrial and commercial applications. Prototype range development includes a full range of chemical hazards testing to support manufacture, as well as the creation of a “Regulatory Support File” for use by QA departments of pharmaceutical sector customers.
Reaxa has now initiated a £500,000 scale-up project for Ni EnCat beads, which is being rolled out through 2010. To date, multiple batches have been produced at 20kg scale in a pilot plant, with kilo-scale samples now available for customer trials.
Production of ‘consistency’ batches are under way at an intermediate 150kg scale and full-scale batches of Ni EnCat at 6,000 litres plant scale are due to be completed by the end of 2010 for commercial supply.
In summary, selection of the right catalyst is critical in identifying process gains and cost savings in commercial production. This means not only obtaining the optimum conversion, but also achieving the simplest, most efficient overall process with the lowest total cost of production.
Recent work with Reaxa’s Ni EnCat has shown the following gains can be achieved compared with standard sponge-type nickel catalysts:
- Lower process cost and less material and solvent usage – reducing metal usage and product contamination by catalyst residues eliminates down-stream purification by solvent washing;
- Reducing liquid effluent and solid waste requiring incineration lowers energy use and potential for CO2 or other atmospheric pollution.
With the imminent scale-up project, Reaxa’s Ni EnCat polymer-immobilised catalyst system is set to deliver real process improvements and cost benefits across a wide variety of chemistries supporting commercial processes in pharma production.
