Attempts to protect the population from the contagious herpes virus have been hindered by its ability to hide from the body’s immune system. The biotech company BioVex explains how its new vaccine addresses this problem
At least 45 million people over the age of 11 in the US have been infected with HSV-2 – Herpes simplex virus type 2 – the agent that causes genital herpes.
Furthermore, the rates of infection increased by 30% between the late 1970s and the early 1990s. In addition, patients with compromised immune systems can experience more severe complications.
One in 10 adults carries HSV-2, though many do not know they are infected as they have mild symptoms. It can be highly contagious, and while condoms and antiviral drugs reduce the risk they do not eliminate transmission. There are antiviral drugs that can reduce symptoms, but they do not clear the virus, which remains dormant or latent in the body after infection.
The economic burden of genital HSV infection and the resulting complications has been estimated to exceed $1bn annually in the US alone. HSV-2 is therefore an important target for vaccine development in three areas:
1. Protection of the sexual partners of those known to be infected with HSV-2;
2. Protection of the general population at large, potentially by mass vaccination of the adolescent population; and
3. Potential reduction in the rate of recurrence in existing HSV-2 carriers, which may also reduce the level of asymptomatic shedding and hence virus spread through the population.
While candidate vaccines have been developed before, they have failed to produce good protection, in part because of HSV-2’s ability to hide from the body’s immune system. BioVex, a privately owned biotechnology company, has designed the new Immuno-VEXHSV2 vaccine to address this problem. It is based on a live but weakened (attenuated) version of HSV-2, which has been engineered to silence five genes that help the virus to evade the immune system.
ImmunoVEXHSV2 expresses all but five of the approximately 80 HSV-2 proteins, maximising the extent of the anti-HSV immune response that is generated. This should increase the probability that the vaccine is able to stimulate an immune response capable of preventing infection.
The construction of the live attenuated virus was achieved by sequentially deleting five genes (vhs, ICP47, UL43, ICP34.5 and US5) known to encode proteins that are involved in host immune evasion. Aftr the deletion of each gene, the virus was put through multiple purification steps. ImmunoVEXHSV2 was then evaluated in the standard preclinical models of genital herpes, in which it completely prevented disease, suggesting that Immuno-VEXHSV2 may be more potent than previous potential HSV-2 vaccines.
enveloped virus
All herpes viruses are enveloped and consist of a large double-stranded linear DNA genome that is packaged in a nucleocapsid approximately 100nm in diameter – an amorphous structure known as the tegument that contains virus proteins and is important in the early stages of the infection process – and an envelope containing viral glycoproteins.
Figure 1 shows the structure of a herpes virus. The envelope contains 12 viral glycoproteins, some of which are essential for cell entry. The size of the complete virion is approximately 120nm. The non-integrating double-stranded DNA HSV-2 genome is approximately 154kbp long and has a high GC content (69%).
The large enveloped HSV-2 virus can infect both dividing and non-dividing cells. So while HSV-2 is good at infecting and replicating in cells, there were a number of manufacturing challenges in producing a live attenuated HSV-2 vaccine that had to be resolved before clinical trials could commence.

Fig. 3: The Mustang Q unit uses a membrane whose large internal surface area offers a high dynamic capacity for large biomolecules compared with conventional beads with diffusive pores
First, attenuated viruses can be more challenging to grow: the removal of genes can reduce the ability of the virus to infect and replicate, and viral growth sometimes requires an engineered producer cell line to compensate for the deleted genes.
Second, as HSV-2 is a large enveloped virus, the virus envelope can be fragile and sensitive to shear forces during purification. The virus envelope is hydrophobic and can cause the virus to stick non-specifically to purification equipment, to aggregate and to bind to cellular debris and DNA, while the size of the virus provides challenges when using a 0.2µm filtration step. During the research phase, laboratory purification methods based on sequential centrifugation steps can produce sufficient stocks of virus to support preclinical studies but they are not suitable for producing large scale quantities of a high purity vaccine that is suitable for mass vaccination.
Initial purification development focused on traditional viral bioprocess methods. Figure 3 shows a typical viral purification process using ultrafiltration and two sequential chroma-tography steps. The process took up to four days to perform, with non-specific binding and aggregation reducing the yield at each step. This process achieved the target of a high purity virus preparation with consistent particle size distribution and low levels of impurities; however, the overall process yield was unacceptable and not cost-effective.

Fig. 4: A range of sizes of membrane chromatography units
Provided compliments of Pall Corporation. Copyright Pall Corporation
During the process development phase a single-use disposable membrane chroma-tography unit, Mustang Q, became available. The Mustang Q unit is composed of 16 layers of PES membrane (0.8µm) that have been coated with charged hydrophilic quaternary amine monomers. The large pore size permits access by large biomolecules to all of the binding sites on the membrane by direct fluid convection. Furthermore the large internal surface area (Fig. 3) produces a very high dynamic capacity for large biomolecules in comparison with the conventional beads with diffusive pores.
membrane chromatography
This high binding capacity can be achieved using high flow rates in all steps of chromatography without loss of resolution or dilution. Membrane chromatography has several potential benefits for the large-scale purification of live virus over conventional chromatography, including cost, ease of use, binding capacity, volume of buffers used and flow rates. The ability to utilise lower elution volumes with membrane chromatography also permitted higher concentrations of virus to be achieved in the final formulation.
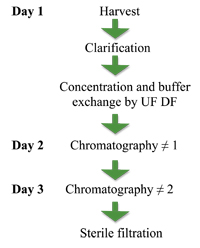
Figure. 5: Classical virus purification process
Membrane chromatography, however, does have its own challenges:
- The binding characteristics of different HSV vaccine constructs have varied significantly.
- As the pores fill up with bound biomolecules, so the back pressure increases and the capacity of the Mustang unit may be reached before all binding sites are full. This can be exacerbated if the viral harvest has not been clarified to reduce turbidity.
- There are regulatory challenges for small companies that choose to be early adopters of new process technology.
The production of each batch of Immuno-VEXHSV2 begins with the preparation of a large batch of Vero cells from the master cell bank. The Vero cells are then infected with a small amount of virus from the viral seed bank. Four days later the infected cells are harvested (Fig. 6) and the HSV-2 virus separated from the process contaminants using a Mustang Q filter.
A 0.45µm filtration step is performed prior to loading the Mustang Q unit to minimise the turbidity of viral harvest that is being applied to the membrane. The virus binds to the Q membrane and is eluted with a high salt concentration. The eluted virus is then de-salted into the final formulation buffer and finally filtered using a 0.2µm filter. The final highly purified virus preparation can then be aseptically filled and, to date, the final formulation has shown good stability of more than three years.
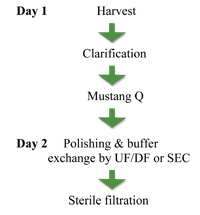
Figure 6: The GMP process based on membrane chromatography
A number of quality control tests were developed at BioVex to test the various physico-chemical and biological properties of the purified ImmunoVEXHSV2 vaccine to demonstrate the identity, potency, safety, purity and impurity levels of the new vaccine. There are particular challenges in demonstrating the viral safety and absence from adventitious viruses when the product is a live and fragile virus.
ImmunoVEXHSV2 has been extensively characterised using growth curves, buoyant density gradients, restriction mapping, size exclusion chromatography, western blotting and epitope mapping. Batches have also been demonstrated to retain sensitivity to antiviral agents such as acyclovir. Finally, the master viral seed bank has been fully sequenced. These characterisation studies will form a solid basis for future process scale-up and process improvement as the vaccine progresses to later clinical phases.
A Phase 1 clinical study is taking place in the UK at the Chelsea and Westminster Hospital in London, where dosing commenced earlier this year. This initial Phase 1 trial is evaluating whether the virus is safe in healthy volunteers, and whether it generates a good immune response. If the results are positive, it will go on to be tested for efficacy on the partners of people with genital herpes.
It should be clear early next year if this novel and rationally designed vaccine has the potential to reduce some of the misery caused by genital herpes and to help minimise the economic impact of HSV-2 infections. Membrane chroma-tography is already proving useful in the purification of live virus at both lab scale and in larger scale production in GMP facilities.
