Water for Injection (WFI) is an essential utility in pharmaceutical and biotech production, with quality standards among the strictest in the industry. The choice of distribution system has a direct impact on process safety, product quality, and the overall operational efficiency of the facility. A well-designed distribution loop helps minimise microbiological risk, optimise energy and resource use, and ensure compliance with global regulations.
By applying advanced technology—both in hot and cold systems—manufacturers can reduce operational costs, lower environmental impact, and maintain critical water purity across highly regulated environments. This article compares both approaches and offers practical insights for implementation.
Hot WFI distribution:
The industry’s traditional approach
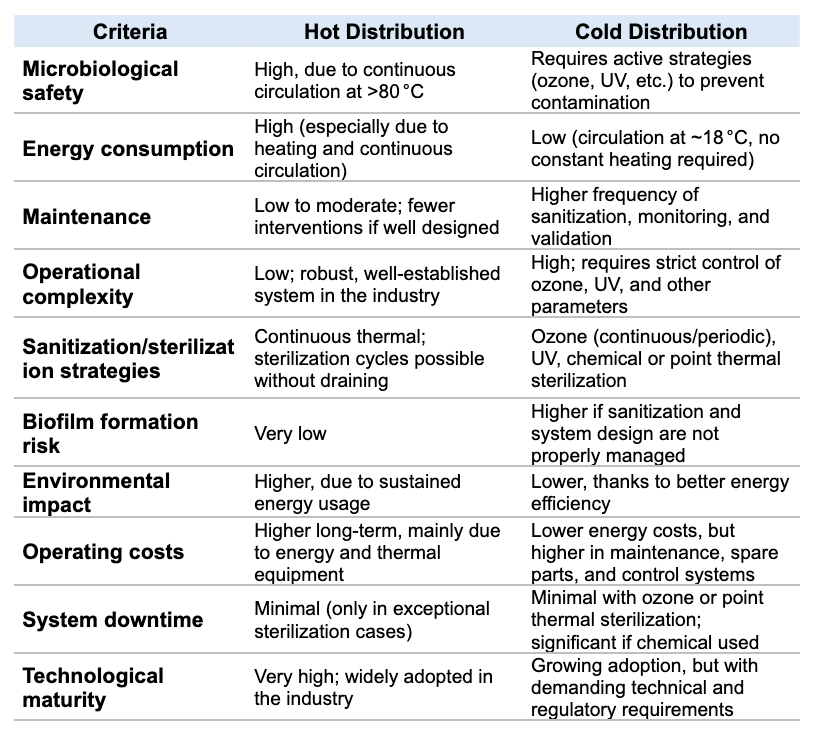
Hot WFI distribution has long been the standard in pharma. This system relies on continuous circulation of water at temperatures above 80°C, keeping the loop permanently sanitised and microbiologically safe.
WFI is typically generated by a distillation system, which accounts for most of the energy usage. Maintaining high-temperature circulation adds further energy demands, making operating cost one of the primary drawbacks of this setup.
Temperature maintenance in the loop
The loop must be designed to sustain the >80°C temperature consistently. This requires thermal control and proper insulation to prevent heat loss and inhibit microbial growth.
Sterilisation when contamination occurs
Although continuous circulation above 80°C provides strong microbiological protection, systems must be equipped to run sterilisation cycles above 121°C when needed. This is achieved by pressurising the storage tank to superheat the WFI in the loop—without requiring pure steam.
Key benefits:
- No need for additional pure steam systems or expensive steam traps.
- No loss of WFI or need to drain the system—saving both cost and product.
- A fast, effective response in case of microbial contamination.
While energy-intensive, hot distribution remains a trusted, robust method in pharmaceutical manufacturing. However, current trends aim to optimise these systems to reduce their environmental impact without compromising microbiological safety or regulatory compliance.
Cold WFI distribution:
The energy-efficient option
Cold WFI distribution provides a lower-energy alternative, delivering WFI at ~18°C from specialised cold generation systems. These systems function similarly to Purified Water (PW) units, but with enhanced capabilities to meet the microbiological and regulatory requirements of WFI.
Cold generation significantly reduces energy use, but it demands more frequent maintenance to maintain WFI quality according to pharmacopoeial standards.
Filtration and treatment processes in cold WFI generation systems to ensure microbiological safety
Cold WFI generation systems rely on advanced purification methods:
- Ultrafiltration: Highly effective against microorganisms and endotoxins, though it requires continuous maintenance.
- Reverse osmosis: Removes remaining impurities and ensures quality water output.
Microbial control: sanitisation and sterilisation methods
To maintain microbiological control in cold WFI loops, various control strategies are applied:
- Ozone sanitisation
- Continuous: Ozone circulates while the system is in use, preventing biofilm formation. UV lamps remove residual ozone before water reaches the points of use.
- Periodic: Higher ozone levels are used during scheduled downtime for deeper disinfection.
- Thermal sterilisation
The system can also superheat WFI to over 121°C using tank pressurisation. This acts as a backup during microbial contamination events or as an alternating sanitisation method.
- Chemical sterilisation
Certain cases may call for chemical sterilants, which provide a deep system cleaning. While effective, they can be aggressive to system materials and require lengthy rinsing procedures, leading to extended downtime.
System design and risk management
Well-engineered design is key in cold WFI loops to prevent stagnation and biofilm risks. Considerations include:
- Using corrosion- and contaminant-resistant materials.
- Ensure continuous flow to avoid stagnation points.
- Real-time monitoring of ozone and other disinfectants.
Although cold WFI distribution offers significant energy savings, it requires rigorous maintenance and effective sanitisation strategies to maintain water quality for pharmaceutical production.
Direct comparison: Hot vs. Cold WFI Distribution