Bachem, a Swiss pharmaceutical company, wants to build a new future in oligonucleotides, focusing on innovation as the critical driver to address increasing demand from the market – as well as more stringent regulatory authorities.
As a result, Bachem has been innovating with mass spectrometry (MS) testing methods for the chemistry, manufacturing and control (CMC) development of oligonucleotides.
The challenges facing oligonucleotides
Oligonucleotides are a new therapeutic modality and so face plenty of regulatory challenges, which can present issues for agencies and pharmaceutical companies. In recent years, the chemical modifications added to oligonucleotides have become more complex and highly modified.
This adds further challenges in the separation of impurities as well as in the development of analytical methods for characterisation.
As this complexity grows further, many analytical methods are unable to accurately characterise the oligonucleotide APIs and identify impurities. Thankfully, there is a solution: high-resolution mass spectrometry (MS) testing.
Although Bachem used MS for the precise analysis of peptide API, the company has seen an opportunity to develop similar testing for oligonucleotides.
What is mass spectrometry (MS)?
Mass spectrometry (MS) is an analytical testing technique that measures the mass of a molecule(s) based on the mass-to-charge ratio of ions. The results constitute the "mass spectrum," which is the exact molecular weight of a molecule.
MS is also used to clarify the chemical structure of a molecule. This can be used to characterise complex oligonucleotides, solving the current issues of identification.
Originally used within the peptide sphere, ultra-high-resolution MS was used successfully for the characterization of peptides at Bachem. MS was then adapted due to the growing relevance of oligonucleotides to their product development as well.
Bachem has collaborated with Bruker to develop a new deconvolution tool, simplifying the spectrum from convoluted (left) to clear and straightforward (right), as seen below.
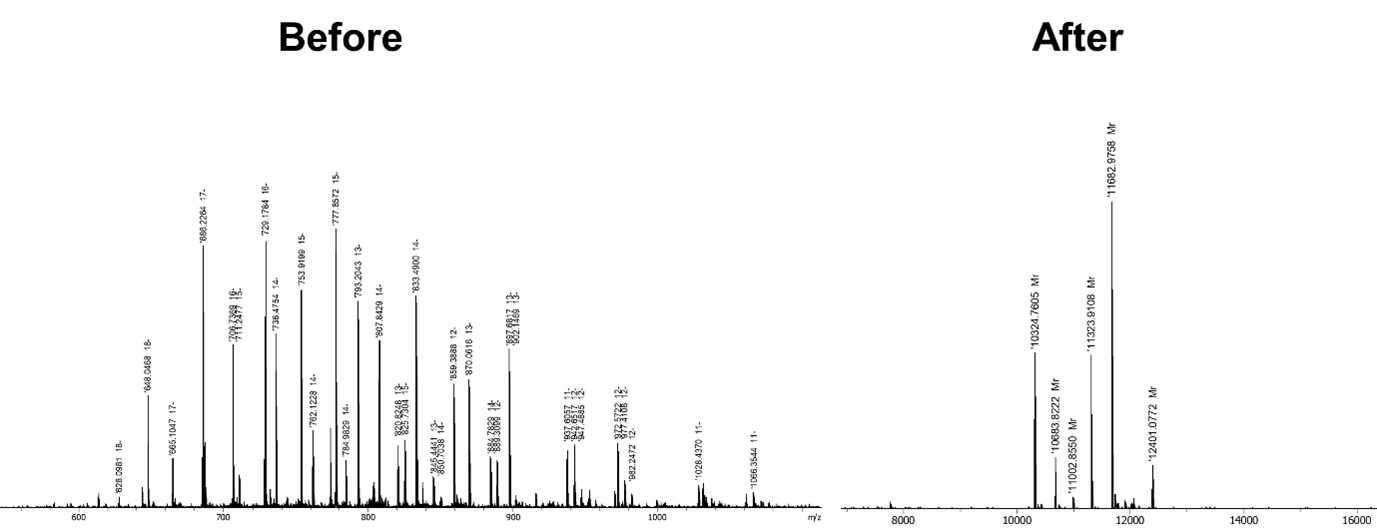
The new deconvolution tool developed by Bachem and Bruker can also be used to identify and quantify impurities, saving time with the potential for future automation.
How MS supports the CMC development of oligonucleotides
Regulatory authorities set strict requirements on impurity levels and their identification, which poses a big challenge for manufacturers when producing an oligonucleotide API.
Therefore, having a robust and reliable process to identify impurities is critical in CMC development. This is why MS is an ideal tool for peak purity analysis. With this approach, it’s possible to identify impurities in oligonucleotide APIs.
This is particularly helpful when identifying common side reactions in oligonucleotide synthesis. Not only does the MS method identify the side reaction, but also the position in the sequence, making it possible to detect and prevent.
Accurate and fast sequence identification
MS is also an effective way to confirm the sequence of an oligonucleotide. Usually, the sequence is confirmed by using chromatographic techniques (LC) coupled with MS equipment.
However, this presents issues for regulatory authorities when it comes to inspection. Bachem’s MS methods are much easier and faster to validate, saving time and improving practicality.
There are some challenges that come with MS testing, such as the signal intensity tends to be lower owong to the size of the oligonucleotides.
Accuracy and precision are key, which is why Bachem is developing a strategy using electrospray ionisation, which directly infuses the oligonucleotide into the ESI source.
Summary
Oligonucleotide-based drugs are of huge importance, and finding the most effective methods for fast approval for pharmaceutical companies is key.
Bachem’s development of precise, ultra-high-resolution MS testing enables the characterisation of the oligonucleotide API as well as the related impurities. This helps agencies and pharmaceutical companies to fast-track their API, helping improve the oligonucleotide market as a whole.
