Launched in June 2009, the F3 Factory project is a four-year €30m public/private sector initiative that is seeking to enhance the EU chemical industry’s competitive position through the development and implementation of faster, more flexible and efficient manufacturing technologies.
As a key, large-scale demonstrator project, F3 Factory is seeking to combine the flexibility of batch manufacturing with the efficiency of large-scale continuous manufacturing to establish the ‘factory of the future’. It will deliver ‘plug and produce’ modular continuous chemical production technology, capable of widespread implementation throughout the chemical industry, to which intensified process equipment can be connected to deliver cleaner, more efficient manufacturing.
The F3 Factory consortium consists of 25 industrial/academic partners from nine European Union Member States that are crossing competitive boundaries to create a new paradigm in sustainable production methodology. The consortium comprises highly complementary skills/expertise and a critical mass of resources from a broad spectrum of industrial, scientific and technological areas required to address the overall objectives of the project.
Industrial applications targeted to demonstrate the F3 Factory concept include pharmaceutical intermediates, solvent-free polymers, speciality surfactants and materials from renewable resources, though it is intended that the technology will be suitable for a broad range of applications.
The seven industrial partners in the consortium – Arkema, AstraZeneca, BASF, Bayer Technology Services, Evonik, Procter & Gamble and Rhodia – will each demonstrate the effectiveness of the F3 Factory concept by operating demonstration processes for their respective product areas on an open access Backbone facility (INVITE) at Chempark, Leverkusen.
Business drivers for standardisation and modularisation: To meet the economic and ecological challenges faced by the European chemical and pharmaceutical industries, significant improvements in process efficiency and flexibility are needed. Through standardisation and modularisation of process equipment, it is expected that industry can achieve a significant decrease in overall capital expenditure.
implementing modular plants
Furthermore, the successful implementation of modular production plants will increase the competitiveness of manufacturing costs for small- to medium-sized production facilities, which – with the growing trend towards production of specialities – is becoming increasingly important.
In place of larger world-scale plants, distributed small-scale plants that are considerably more flexible and available with shorter lead-times can be built. Implementing this new ‘smart scale’ technology will sustainably accelerate time-to-market by reducing project duration from product development to production.
Initial assessments by the project partners have indicated that there is a potential for the EU chemical industry to achieve an estimated b4bn in cost savings at the same time as driving opportunities to generate new business and thereby strengthen the sector’s overall competitiveness.
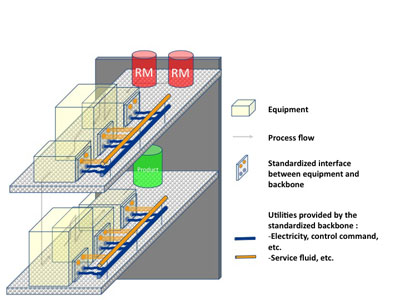
Figure 2: The F3 Factory Plug and Produce concept
F3 Factory standards to enable “Plug-and-Produce” manufacturing: The F³ Factory is based on a ‘Plug-and-Produce’ concept (see Fig. 2), which consists of:
- Process Equipment Assembly (PEA) – the smallest modular element, a PEA wraps process equipment items associated with one or more process tasks.
- Process Equipment Containers (PEC) – this is the superstructure that holds one or more docked PEAs in position and provides them with required services.
- Backbone Plant – this is equipped with standardised connections to enable one or more docked PECs to interface with typical utilities and process control equipment.
Process Equipment Assemblies will be supplied with standardised connections to be easily mounted into Process Equipment Containers. Plant services and ancillary equip-ment will be modularised and standardised by suppliers according to ranges of operating conditions.
The flexible, rapid mounting and dismounting of PEAs and PECs enabled by these standard interfaces should offer:
- a higher capital utilisation rate for the backbone facilities and
- a higher return on capital investment.
Shortening innovation lead times: The backbone facility for the F3 Factory will be provided by INVITE (INnovation, VIsion, TEchnologies) – a 50/50 joint venture established in 2010 between TU Dortmund University and Bayer Technology Services.
The INVITE backbone facility – which will be embedded in the infrastructure of Bayer Technology Services at the Chempark site, Leverkusen – will allow three different process equipment containers (PECs) to be developed, tested and operated in parallel. Additional facilities include laboratory space, offices and a lecture room (see Fig. 1).
INVITE will take the F³ Factory vision beyond the EU project by providing an open research centre for developing and demon-strating future manufacturing technologies.
This combination of joint research by industry and academia with access to a full industrial infrastructure is intended to shorten innovation periods significantly.
Transforming batch to continuous in pharmaceutical production: In recent years, the fine chemical and pharmaceutical industries have come under increasing pressure to drive efficiencies at all stages of production. The main drivers for this include:
- increased global competition
- enhanced efforts for the discovery of new active compounds
- the need for a more sustainable production structure
- rising energy and raw material costs
- the maturity of ‘classical’ chemistry
The pressure resulting from these drivers is forcing companies to seek new ways to optimise production processes in the short term and focus resources on analysing what benefits can be realised in the mid-long term. Clearly quasi-continuous technologies will be of vital importance to this approach and the F3 Factory concept could be a key enabler for this new manufacturing paradigm.
case study 1:
Bayer Technology Services (BTS) is leading an industrial case study that seeks to demonstrate the F3 Factory modularisation concept for pharmaceutical intermediate production. The aim is to assess the potential for driving cost efficiencies and manufacturing flexibility by transforming pharmaceutical intermediate processes from batch to continuous.
Previous studies within BTS have demonstrated that conversion and production costs favour continuous processes. It is also generally recognised that inherently lower inventories are achieved if all processing steps are performed on one site. Novel chemical routes in continuous processing can often allow for the elimination of processing steps. In addition, novel process-intensified apparatus enable cheaper production routes that would not normally be considered in batch processing.
Several single stage solutions have been proven for a number of internal Bayer examples and there are other successful examples accessible in literature.
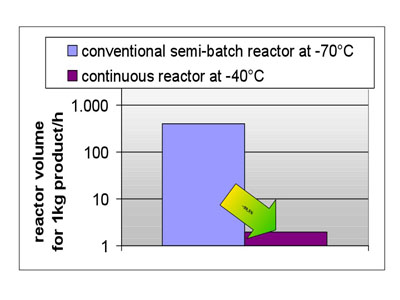
Figure 3: A benefit of continuous production is a large reduction in reactor size
On average it was found that by transforming a dedicated process from batch to continuous a 10–25% reduction in operating costs and approximately 20% reduction in investment costs can be achieved. Telescoping a process through two reaction steps without isolation allows for total reductions of up to 60%. A simple transfer of elaborated batch protocols to continuous processing will, therefore, result only in a realisation of a share of the maximum benefit possible (see Figures 3 and 4).
These challenging process efficiency aims (increased yield, reduced investment/operating costs, decrease in QA efforts, increase in the degree of automation of all manufacturing steps) have to be evaluated for specific processes and this will be enabled by the F³ Factory case study.
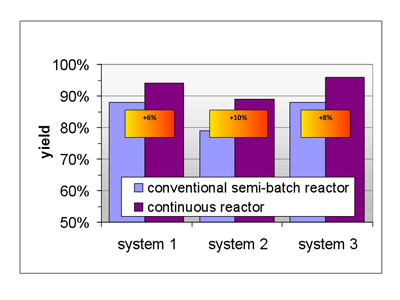
Figure 4: Continuous reactors could also provide increased yields
case study 2:
As a key industrial partner in the project, AstraZeneca is leading a study to demonstrate the flexible, continuous production of new pharmaceutical materials for toxicological studies. Working with the academic partners KTH Institute of Technology, Denmark Technical University, Newcastle University and Karlsruhe Institute of Technology, as well as an industrial partner, Britest, this sub-project is seeking to develop and validate a generic methodology for the preparation of pharmaceutical intermediates.
One of the biggest challenges facing the international pharmaceutical industry is the ability to offer a flexible response to the production of new materials for toxicological studies (in vivo, in vitro).
The F3 Factory approach to providing a ‘one size fits most’ process synthesis for the production of intermediates for campaign two material, is seen as a key step in building a fast and flexible response to this need and reducing production costs in this area.
AstraZeneca currently manufactures new materials for toxicological studies in traditional batch processing equipment at the 10–100 litre scale.
The company believes that a continuous process capability at this scale will add significant production flexibility and enable it to assess new materials more quickly and at a lower cost. As such, the company is seeking to develop a continuous manufacturing approach for a range of typical pharmaceutical intermediates (see Figure 5).
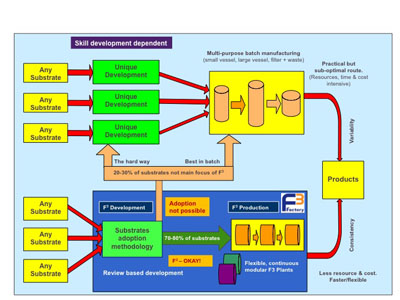
Figure 5: F3 Factory transformation methodology could free-up resources to focus on later stage development
It is increasingly felt that continuous manufacturing could also be applied to the final stage active pharmaceutical ingredient synthesis – and, therefore, continuous technologies for work-up in this area are of major interest.
If this industrial demonstrator project proves successful, the vision is that up to 80% of AstraZeneca’s pharmaceutical intermediate processes will follow the F3 Factory transformation methodology, freeing up resources to focus on later stage development. This will result in significantly less abortive effort in the development stages of a new drug product. The F3 Factory methodology will also support a concerted move from batch to continuous production.
A key barrier to overcome is purely a mindset issue of ‘we’ve always done things this way, so why change’ and ‘if it isn’t broke why fix it?’ In recent years there has been some progress in this respect across the pharmaceutical industry but there is still more progress to be made. There are also QA issues to overcome with regard to continuous processing versus batch manufacture, e.g. how to define ‘in spec’ and ‘out of spec’ material.
For further details on the F3 Factory project contact Dr Sigurd Buchholz, Project Coordinator, Bayer Technology Services: email sigurd.buchholz@bayer.com; or go to www.f3factory.eu.
