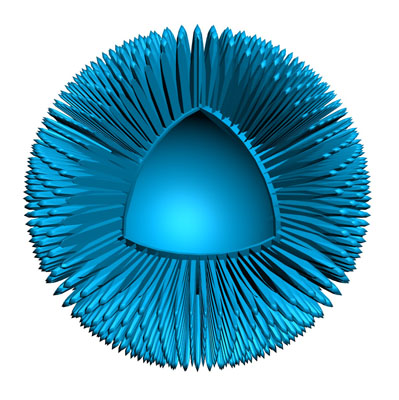
Says Nigel: “N4 has developed a breakthrough delivery system technology that’s designed to work with DNA and RNA, amongst other things, and deliver that payload into cells. It’s a tiny, hollow, mesoporous nanoparticle made from silica."
"What’s unique about it is that Nuvec silica nanoparticles have an irregular (spiky) surface structure – coupled with polyethyleneimine (PEI) – that simply and effectively traps and protects nucleic acid (such as mRNA/pDNA) as it travels to the cells. Once inside the cell, it is released to activate the immune system. Nuvec is also a natural adjuvant, so it attracts a large number of innate immune cells which, in turn, leads to more activation of the adaptive immune system (T and B cells), thus increasing the level of immune response against the target cancer cells.”
“Manufactured at a size of 180 nm,” he adds, “we basically functionalise the silica particles, which changes the charge of the particle and makes it positive … so it can then bind the DNA or RNA payload. Keeping that positive charge is crucial for cellular transfection. Fundamentally, the spikes trap and protect the nucleic acids within the structure. Nuvec does not seek to totally encapsulate the DNA or RNA however it binds and protects sufficient amounts to deliver good transfection. Its high surface area, owing to the spikes, allows for high levels of material to be loaded onto the particle.”
Once the DNA/RNA, trapped in an endosomal bubble, is inside the cell (entry is via endocytosis), the PEI helps to rupture that bubble and releases the active directly into the cytoplasm. There, the DNA or RNA activates the relevant cells, be those T cells (cancer vaccines) or B cells (viral therapies), and allows them to do their job.
“The other thing that’s really important is that the Nuvec system doesn’t have any unwanted systemic side-effects,” adds Nigel: “We’ve got data showing that the drug remains at the site of injection; it doesn’t produce unwanted inflammatory responses and, very importantly, it doesn’t track to the liver. These are some of the key differences between Nuvec and other systems.”
A brief history
Existing delivery systems, such as lipid nanoparticles, were not originally designed to work with nucleic acids and, although they can produce good immune responses, they can be unstable, difficult to handle and often require an additional adjuvant/immunomodulator to ensure immunogenicity.
They can also cause liver toxicity. Nuvec has been designed specifically to work with nucleic acids, which are the subject of significant research investment in the fields of cancer immunotherapy and vaccines.
The original technology, Nigel explains, is licenced from the University of Queensland in Australia. Researchers there were working with a mesoporous silica particle that resembled a practice golf ball (a hollow particle with pores). “What the university was doing was changing the size of those pores to allow subunit vaccines such as bovine diarrhoea or hepatitis B to actually get inside the nanoparticle."
"Once the subunit vaccine was in there, it was then trapped and slowly released. It was all very well for subunit vaccines but, at the time, it didn’t work with DNA or RNA. Subsequently, they developed a new approach that reduced the pore size to approximately 6 nm and, by changing the manufacturing process slightly, were able to grow and add these spikes onto the structure. The next challenge was working out how to attach something without relying on a covalent bond,” he says.
The solution was to use PEI to electrostatically bind the DNA/RNA to the particles; as such, the bond is strong enough to remain intact during delivery but also weak enough to release the payload in situ.
“The way it’s made, which is important, is quite simple,” explains Nigel: “Pharma companies don’t want to implement complicated manufacturing processes that will ultimately cause their product to fail at the last minute. They want to be able to monitor and control the entire procedure. So, our system basically comprises a series of mixing steps. There’s no complicated machinery or equipment involved, but what’s unique is the way we do those steps and the silica particle that comes out the other end.”
Scale-up and large-scale production
Asked about industrial-scale manufacturing, Nigel assures me that Nuvec has been developed with a scalable process using standard technologies. “We know it can scale-up very easily. We’ve now gone from 0.5 g in the lab to a continuous preclinical manufacturing step of 17 g that takes 4–5 days, but the process that we’re using to make that amount is just a series of vessels. We want to move up to 250 g and, fundamentally, it’s just about bigger vessels,” says Nigel.
“At the moment,” he adds, “we’re talking to some tech transfer partners about expanding into a GMP-capable facility. We don’t need GMP-grade product, just yet, but considering there are about 6 million, billion, particles (or something like that) involved in the 17 g production step, there’s a lot of material to work with; and, if you look at the doses that people are using for DNA and RNA treatments, supplying our particle at the scale required is not going to be a problem.”
Human clinical trials
Studies so far have shown that Nuvec is well tolerated, even at high doses, with no major toxicology findings across in vivo rat/mouse and in vitro PBMC/spleen studies. Recent work has confirmed that the immune response observed with Nuvec was sufficient to produce high levels of antibodies specific for the standard test antigen ovalbumin (OVA).
A high level of antibody production is essential for a vaccine to initiate immunity. The levels of antibodies produced were greater than those of in vivo jetPEI, an industry standard used to deliver pDNA-OVA in preclinical studies. Furthermore, assessing the produced antibody isotypes, it became evident that Nuvec delivers a sufficiently robust immune response and of the type needed for an effective vaccine in the field of oncology and virology.
Further experiments are planned. “What we’re looking to do next is to show not just the immune response, but the efficacy of the particle,” says Nigel. “With OVA, we’ve got the transfection data, the loading data and the immune response data – now we need to show we can deliver efficacy. We’ll also then look at using some other antigens, both in the cancer and viral space. As we believe that Nuvec has the potential to work in both a cancer vaccine, or a prophylactic vaccine for hard to treat diseases, that’s where we’ll be directing our research in the near future.”
Considering the number of antibodies produced and the efficacy of the system, I asked whether Nuvec could be used to reformulate existing treatments, possibly with lower doses, to deliver the same clinical effect.“It’s a good question,” conceded Nigel, “but when we look at DNA and RNA, there are very few existing products with which to make that comparison.” He continues: “Nuvec itself is a natural adjuvant; so, by combining a DNA or RNA candidate with the Nuvec system, we very much hope to see either a bigger response at the current dose or the same response at a lower dose … depending on what the required end result is. So, to answer your question, yes, we see that as a real opportunity … but there isn’t anything on the market yet that we can use as a comparison.”
Unique offering
From what we’ve seen, observes Nigel: “There’s nothing like this, using silica, on the market … and that’s what’s so strong about our patent. Essentially, if you’ve got a DNA/RNA-based active, you’re going to have to go shopping to see what’s out there. You might choose a lipid system, which are great for encapsulation, but not so good for release. There are, indeed, other silica systems that have been designed for other agents, and you can certainly charge them with DNA and RNA; but, in our research, we’ve shown that none of those produce the level of transfection that our spiky system does.”
“It’s that spiky structure that makes our system unique."
He adds: "Yes, there are viral vectors that have a similar structure, but they are very hard to make and have lots of side-effect issues, so people are moving away from them. So, we think we’re pretty unique, both in terms of shape and certainly how we use silica.”
Next steps
Looking ahead, Nigel foresees a rosy future: “The more data we generate, the more it impresses us, so we’re very confident that we can become a key alternative to using lipids. The challenge we face as a company is getting hold of those antigens and working with partners so that they can actually see the results with their own DNA or RNA. At the moment, we are either having to partner with somebody or use model antigens because we don’t own the DNA or RNA we need to bring this delivery solution into both oncology and virology applications.”
Right now, Nigel feels it’s important to spread the word, make more people aware that this technology is available, how it works and ensure that it fits their target profile. “If someone comes to us with a target profile, we can hopefully be part of their preclinical programme, he says, adding: “From a manufacturing perspective, the next step is tech transfer to a GMP facility."
"We don’t want to be a rate-limiting step for anyone who wants to go into clinical trials in 2020. We’ll be relying on our partner to go into the trial because our system will be combined with their product. We want to be ready by then so that they can go into clinical trials at that time.”
Like all great ideas, Nuvec is derived from a combination of factors. The basic science, mesoporous silica nanoparticles for drug delivery, has been around for a few years. “The uniqueness,” concludes Nigel, “is the creativity of the guys at the University of Queensland. They came up with the idea of a structure that would non-covalently bind DNA to a delivery vehicle and effectively transfect a cell. It’s a good example of how you take existing technology and apply creative thinking to come up with something that’s very revolutionary.”
NB: This article will appear in the April 2019 issue of Manufacturing Chemist. A recent digital edition is available online.
