Recently Enzymicals has implemented the recombinant expression for a diverse set of phosphotransferases, mainly carbohydrate kinases, in a cooperation with Merck. The main aim was to improve the availability of enantiopure phosphorylated key metabolites and intermediates via biocatalytic phosphorylation.
An example for an enantiopure phosphorylated metabolite is N-phospho-L-arginine, which is part of the energy buffering system of many invertebrates and also of pathogenic protozoa like Trypanosoma and Leishmania species which cause Chagas disease or leishmaniasis in human.
The highly active and selective arginine kinase from horseshoe crab has been chosen as suitable catalyst for the synthesis of this important metabolite. By a synthetic gene approach, this biocatalyst was recombinantly produced in E. coli. Thus, the overexpressed arginine kinase enabled a simple and straightforward synthesis of the enantiopure metabolite N- phospho-L-arginine in gram quantities in only one step. So a tedious and unselective chemical five step synthesis was replaced and the lack of availability for this metabolite closed. [1]
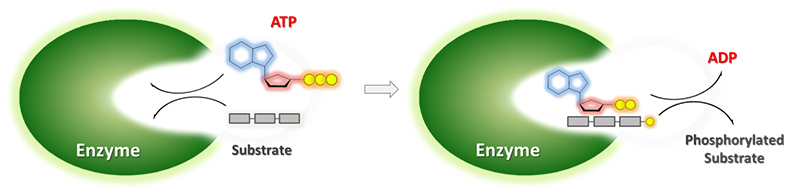
Another important phosphorylated key intermediate is shikimic acid-3-phosphate (S3P). This intermediate is part of the shikimate pathway, which is absent in mammals, but is essential for the synthesis of aromatic amino acids and of almost all other aromatic compounds in algae, higher plants, bacteria and fungi. What is more, it is the cosubstrate of the EPSP synthase, which is the prime target for drugs and herbicides like glyphosate [2].
In order to synthesis this important intermediate by biocatalysis, the Enzymicals team produced the stable and selective shikimate kinase AroL in E.coli. With the help of this biocatalyst, the traditional chemical multistep synthesis could be replaced by a simple one-step enzymatic phosphorylation in good yield, high purity and at gram scale. [3]
Currently, there are 10 kinases in the internal portfolio of Enzymicals. A selection of three kinases are incorporated in the newly updated catalogue.
Please contact us for questions, offers and/or orders at E: info@enzymicals.com.
You are welcome to visit Enzymicals at stand 2D78, CPHI Europe, 4-6 October 2016, Barcelona
References[1] eBook of Abstracts of 8th International Congress on Biocatalysis 2016, L1-6 Arginine Kinase-Catalyzed Phosphorylation of L-Arginine (#306)
[2] Daniell, Henry, and Christine D. Chase, eds. Molecular biology and biotechnology of plant organelles: chloroplasts and mitochondria. Springer Science & Business Media, 2007.
[3] eBook of Abstracts of 8th International Congress on Biocatalysis 2016, P1-48 Shikimate Kinase-catalyzed Phosphorylation of Shikimate (#308)




