The focus of Enzymicals is to provide integrated solutions for biocatalytic applications.
The company is dedicated to design, development and the implementation of cost-effective, sustainable and scalable chemo-biocatalytic routes.
Enzymicals offers recognised expertise in the application of enzymatic processes for the synthesis of complex chemicals from the laboratory up to an industrial commercial scale.
The application of Enzymicals’ recombinant pig liver isoenzymes (rPLE) enables the synthesis of novel, optically pure diastereomers of substituted proline, which show activity as anti-Mannich catalysts.
Additionally, Enzymicals’ rPLE is the key for successful production of a previously unavailable bicyclic carboxylic acid ester, with four stereo centres, which is an interesting intermediate for the pharmaceutical industry.
The asymmetric Mannich reaction is an important carbon-carbon bond forming reaction for the synthesis of β-amino carbonyl compounds. The demand for Mannich reactions that selectively provide anti- or syn-products with high enantioselectivity is high.
Besides, proline which gives easy access to syn-products pyrrolidine derivatives catalyse enantioselective anti-Mannich reactions; however this type of reaction is rare.
Professor Masterson's Group of the University of Southern Mississippi has developed a new synthetic route to provide two diastereomers of 5-benzyl substituted Cα-methyl-β-proline in their optically pure form, from a malonate diester using Enzymicals rPLE. For the synthesis of the new anti-Mannich catalyst a new stereoselective cyclisation strategy was utilised.1
Both diastereomers of the 5-benzyl-Cα-methyl-β-proline analogue were tested for catalytic activity in the Mannich reaction between α-imino ester and isovaleraldehyde. The 5-benzyl substituted (3S,5S)-Cα-methyl-β-proline (figure 1), proved to be an efficient anti-Mannich catalyst providing optical pure anti-products.
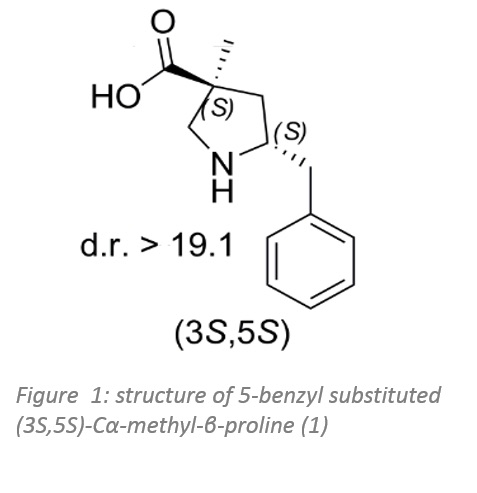
Further steps of optimisation include the chemo-enzymatic synthesis of the organocatalyst and the final anti-Mannich reaction.
The second example shows the utilisation of a scaled-up biocatalytic method developed by Enzymicals as basis for the production of a previously unavailable intermediate, (1R,2S,4S,5S)-4-hydroxy-7-oxo-6-oxabicyclo [3.2.1] octane-2-carboxylic acid methyl ester (figure 2).
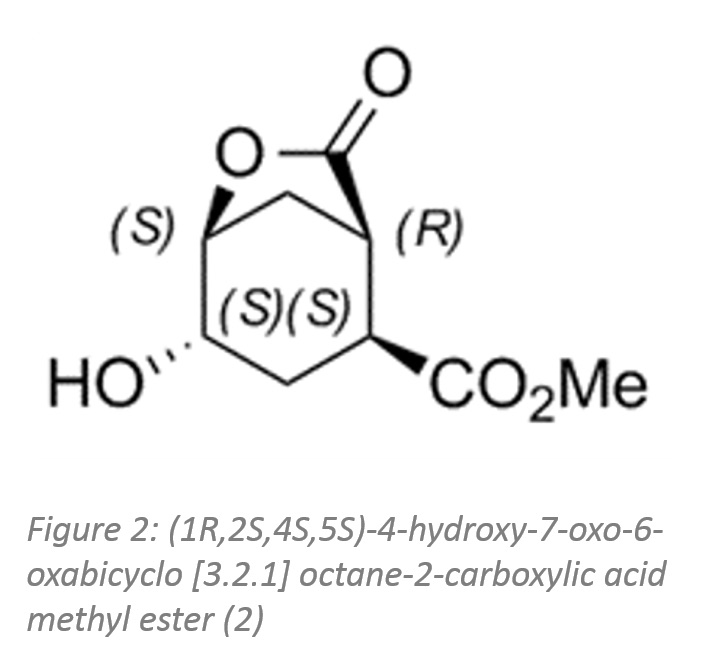
The four stereo centres of this bicyclic compound are a challenge for classical chemical synthesis. The synthesis of this stereo pure methyl ester was enabled by a combination of chemical and biocatalytic steps.
The chemoenzymatic process was originally developed for the production of (1S,2R)-1-(methoxycarbonyl) cyclohex-4-ene-2-carboxylic acid (figure 3) by rPLE in an optimised enantioselective sequential multistep one pot reaction.
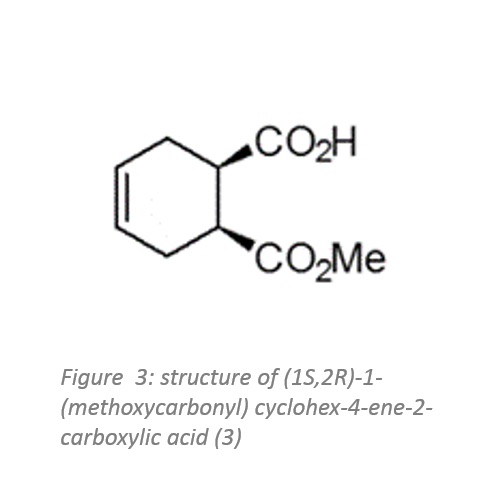
Herein an optimised esterification protocol, starting from the economically favoured meso-anhydride, was established and combined with the highly selective ECS-PLE06-catalysed desymmetrisation reaction.2
The enantiopure compound (figure 3) serves as starting material for the following oxidation and rearrangement reaction leading to ring formation. After optimisation of the process and the downstream procedure, it was possible to produce the new enantiopure intermediate in multi-hundred-gram scale.
Enzymicals believes the combination of biocatalysis with classical chemical synthesis and other stereo-selective techniques can bring a competitive advantage to research, development and production.
The company manufactures a range of chemicals as chiral building blocks, intermediates and specialty chemicals and offers custom synthesis on request.
Enzymicals offers a broad selection of recombinant enzymes suitable for research, development, production and diagnostics as well as a tailor-made protein expression and optimisation services.
Enzymicals will exhibit at CPhI 24–26 October, Frankfurt, hall 6.0, booth F72.
References
- H.K. Kotapati, et al., “Diastereoselective hydrolysis of branched malonate diesters by Porcine Liver Esterase: Synthesis of 5-benzyl substituted Cα-methyl-β-proline and catalytic evaluation,” European Journal of Organic Chemistry, (2017).
- P. Süss, et al., “Chemoenzymatic Sequential Multistep One-Pot Reaction for the Synthesis of (1S,2R)-1-(Methoxycarbonyl)cyclohex-4-ene-2-carboxylic Acid with Recombinant Pig Liver Esterase,” Org. Process Res. Dev., 19(12), 2034–2038, (2015).
