Polyol erythritol is becoming a valuable diluent option for chewable tablets thanks to its taste-masking properties. Liesbeth Meeus, Application Centre Leader for Cargill Pharma & Personal Care EMEA, describes new wet granulation techniques using Zerose erythritol
The modern pharma industry is challenged to reduce costs, adjust production volumes, provide more tailored solutions and defend intellectual property. In the face of this, excipients are finding themselves with a more important role.
Clearly, excipients must still enable the effective delivery of active pharmaceutical ingredients. However, excipients with additional functional properties, for example polyols, can be tailored as ‘innovation tools’ to underpin new product development, defend the lifespan of existing products, reduce costs and provide new solutions for pharmaceutical manufacturers.
One of the key challenges for both over-the-counter (otc) and prescription medication is user acceptance. Traditional hard tablets can be difficult to ingest, both in terms of their size and the need for an accompanying drink to aid swallowing. In response, tablet manufacturers have worked on reducing the size of the oral dosage form, adopting a more oesophagus-friendly shape and adding coatings to ease peristalsis.
However, there is limited scope to reducing the size of some tablets due to the quantity of active ingredient required for an effective dosage form. In addition, some users are unwilling or unable to swallow hard tablets of any size and instead choose to chew. However, if the tablet contains a bitter active ingredient, the outcome will be unpalatable, which in turn affects acceptance.
In response to this challenge, Cargill has developed a way to improve the palatability of bitter actives in chewable tablets, using excipients from its portfolio of pharma-grade polyols (sugar alcohols). Key among these is Zerose erythritol, which possesses valuable taste-masking properties.
fermentation process
Zerose erythritol is obtained via an enzymatic hydrolysis of starch into glucose, followed by a patented fermentation process, which produces a white, crystalline powder. Erythritol has been consumed by humans for thousands of years as it occurs naturally at low levels in many fruits, mushrooms, and in popular fermented foods such as soy sauce, cheese, wine, beer and sake.
Compared with other polyols, erythritol has some unique properties. For example, it is the only zero-calorie polyol, and has a higher digestive tolerance than any other polyol.
However, the most crucial benefit of erythritol for pharma applications is its valuable taste-masking properties. These can help to make bitter actives taste more acceptable in oral dosage formulations. Evidence of this is clearly seen in numerous studies with food compounds, as illustrated in Figure 1.
Thanks to its taste-masking effect erythritol synergises well with high-intensity sweeteners. It possesses a pleasant-tasting mild sweetness approximately 60–70% that of sucrose, plus its high negative heat of solution provides a strong cooling effect.
Like all polyols, erythritol does not ferment in the oral cavity, and so it is non-cariogenic, again an important attribute for oral dosage forms. Furthermore, erythritol does not produce any glycaemic/insulinaemic response, which means it may help the growing number of pre-diabetic and diabetic people to better manage their available carbohydrate intake.
It provides good heat and pH stability, and again provides a good benefit for pharma through low viscosity, and non hygroscopicity (up to 90% relative humidity) that makes it suitable for moisture-sensitive active ingredients.
Erythritol can be used in solid dosage forms as well as tablets and syrups, such as cough mixtures. One European client is already using Zerose erythritol in a sachet medicine.
excipient and diluent
Despite its potential to improve the acceptance of tablets containing bitter or unpalatable actives through improved taste, erythritol has not found wide use in this application.
One issue has been erythritol’s innate lack of compressibility, which of course has a major bearing on key tablet properties. On the one hand, there is a need to optimise hardness and friability, to help minimise packaging costs; on the other, it is vital for tablets to deliver sufficient and active doses of active ingredient, while still remaining acceptable to the user.
Both of these factors present serious challenges with an uncompressible diluent such as erythritol. But at the same time there could be significant benefits for manufacturers and pharma users if erythritol could be used in tabletting.
With more than 10 years of experience across its wider portfolio of pharma-grade polyols and other carbohydrate-based excipients, Cargill Pharma was well placed to overcome the challenge posed by erythritol.
As the only erythritol manufacturer with a pharma certificate of quality, Cargill’s Pharma Application Centre has used Zerose to investigate how erythritol could operate as a diluent, in the context of a high-shear wet granulation process to alter particle size, and employing binders drawn from other parts of the Cargill Pharma polyol portfolio.
High-shear granulation combines the active powder with a binder solution using a high-speed mixing blade (impeller) and chopper. The process used is as follows:
- Sieving and mixing of drugs and excipients
- Preparation of the binder
- Wet massing by addition of liquid
- Screening of wet mass
- Drying of the wet granules in a fluid bed
- Screening of the dry granules
- Blending with lubricants
- Compression of tablets
The results have been favourable and Cargill Pharma has filed two patents on the use of this technique with Zerose erythritol. In both cases, Cargill has undertaken pilot studies to investigate the hardness of tablets that can be produced using the new, patented techniques.
The first patent, filed in 2008, employs Cargill’s C*PharmSorbidex liquid sorbitol as the binder. The second, filed in 2009, employs C*PharmIsoMaltidex isomalt.
Erythritol granulated with sorbitol
It is valuable to note the difference in powder properties demonstrated by erythritol before and after granulation with sorbitol, as demonstrated by Table 1.
Granulation of the ingredients with a liquid sorbitol binder results in a regular and increased particle size distribution. This has a positive influence on the flow as shown in Table 1 with a lower value of Compressibility Index and Hausner Ratio (based on the relationship between loose and tapped density).
| Table 1: Comparison of powder properties for Zerose erythritol before and after granulation | |||||
| Volume mean diameter (VMD) (µm) | Loose density | Tapped density | Compressibility | Hausner ratio index/td> | |
| Non-granulated Start formulation | |||||
| Granulated with C*PharmSorbidex NC 16205 | |||||
A tabletting trial was undertaken in a typical process (using a Korsch PH103 triple punch rotary press at a tabletting speed of 40rpm), using both the placebo and a paracetamol formulation in the following percentage weights:
- Placebo: 86% Zerose erythritol granulated with 14% C*PharmSorbidex liquid sorbitol
- Active: 43% Zerose erythritol and 43% paracetamol granulated with 14% C*PharmSorbidex liquid sorbitol
The results are summarised in Figure 2. Even for tablets containing 50% of the active (500mg paracetamol) tablet hardness is still at a good level. This was a successful result in terms of hardness and friability.
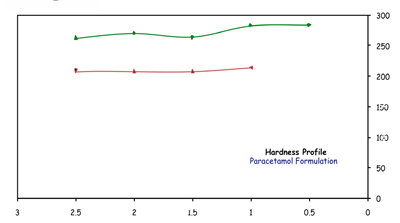
Figure 2: Compression profile of the granulated formulation with liquid sorbitol – with (500mg paracetamol) and without active
Erythritol granulated with isomalt
Building on its initial research, Cargill Pharma made further progress in 2009 by developing a second patented method using C*Pharm-IsoMaltidex isomalt as the binder in place of sorbitol. Because isomalt is non-hygroscopic, like erythritol, the resulting tablets have a low hygroscopic profile, see Figure 3. This in turn introduced the opportunity to produce good-tasting chewable tablets suitable for moisture-sensitive actives.
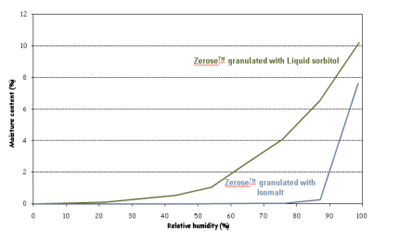
Figure 3: Profile comparing moisture absorption – Zerose with liquid sorbitol and Zerose with granulated Isomalt
In studies of tablets produced using both a placebo and an active ingredient formulation, results were once again promising in terms of dilution potential, as Figures 4 and 5 show.
Here, the placebo formulation included 80% Zerose erythritol and 20% C*PharmIsoMaltidex isomalt; the active formulation included 30% Zerose, 20% C*PharmIsoMaltidex and 50% Vitamin C. Fig. 5 shows the dilution potential profile of tablets with and without active ingredient. It is also worth noting that in Cargill’s studies the placebo formulation tablets achieved a hardness of 196 N, a tensile strength of 4.05 N/m2 and friability of 0.31% at 351mg tablet mass.
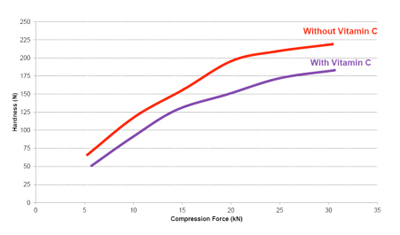
Figure 4: Dilution potential profile of 350mg granulated Zerose erythritol tablets
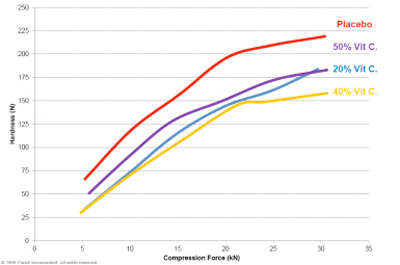
Fig 5: Tablet properties obtained with various different levels of active
Further capability studies are underway to tailor the process for use with specific active pharmaceutical ingredients. And at CPhI Worldwide 2010 Cargill announced details of a new patent application for the use of Zerose erythritol as an excipient in orally disintegrating tablets.
In conclusion, thanks to its unique taste-masking properties, erythritol can enable the creation of effective oral dosage forms containing bitter or unpalatable actives. However, for the creation of good quality tablets, erythritol must be combined with a synergistic binder and undergo specific granulation steps.
Successful techniques to achieve this have been developed by Cargill Pharma in a growing family of patented and patent-pending wet granulation techniques that combine Zerose erythritol with binders such as C*Pharm-Sorbidex sorbitol and C*PharmIsoMaltidex isomalt.
The opportunity is now open for international pharma brands to work in partnership with Cargill Pharma’s Application and Regulatory teams, in the development of valuable new concepts in chewable medication.
