For intensified processes to be scaled-up effectively, operating conditions need to be modelled accurately. To do this in competitive time frames using regular fed batch, this needs to be done in high throughput mode; in 2018, SSB introduced a new version of its ambr technology platform for intensified processing — the ambr 250 high throughput perfusion system (Figure 4).
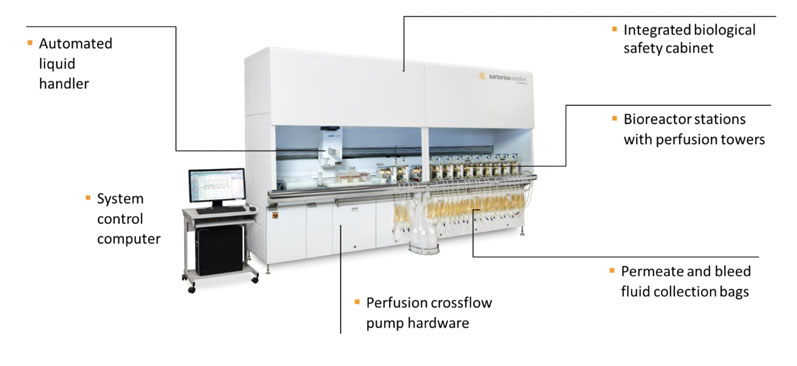
Figure 4: ambr 250 high throughput perfusion system
This system uses up to 24 single-use stirred bioreactors (250 mL working volume) and, because the ambr bioreactors have similar geometries to larger stirred tank bioreactors, help to develop process intensification conditions that can be seamlessly transferred to pilot and manufacturing scale.
To demonstrate the performance of ambr 250 high throughput (HT) perfusion, a biopharmaceutical partner company cultured a cell line expressing a coagulation factor for 30 days in two ambr 250 HT perfusion bioreactors and two 5 L benchtop bioreactors as a scale-down model in alternating tangential flow (ATF) mode.
The crossflow and dilution rates used were within typical operating ranges for ambr 250 HT perfusion; automated bioreactor bleeding was based on daily cell counts to keep the viable cell density within the reference process target range.
The results (Figure 5) showed that cell growth and viability were consistent between ambr 250 HT perfusion replicates and were comparable with those seen in the 5 L benchtop bioreactors, which represented the current scaled down model.3
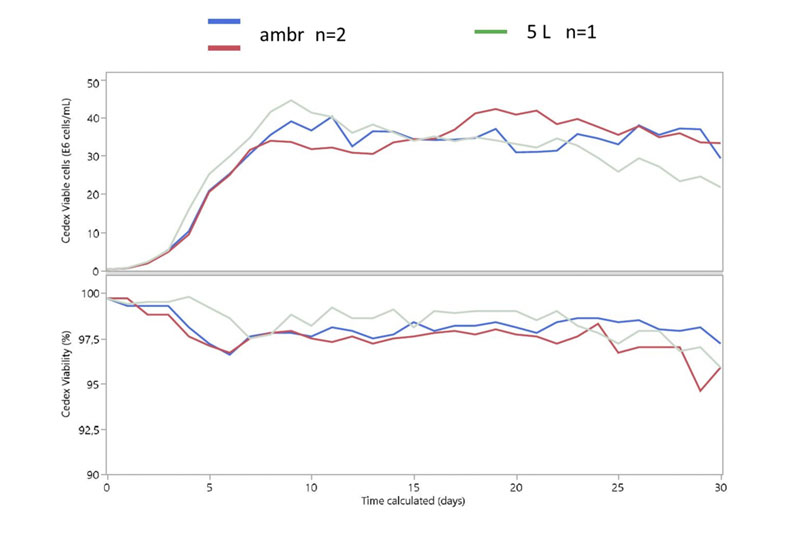
Figure 5: Viable cell density of cells expressing a coagulation factor cultured for 30 days in ambr 250 HT perfusion bioreactors and 5 L benchtop bioreactors
Additionally, four quality attributes were tested (A, B, C, D) each week from the ambr and 5 L bioreactors for three weeks of culture. The results showed that there was good consistency between both bioreactor types (Figure 6), indicating that product quality in an ambr 250 HT perfusion bioreactor is representative of larger scale perfusion cultures.4
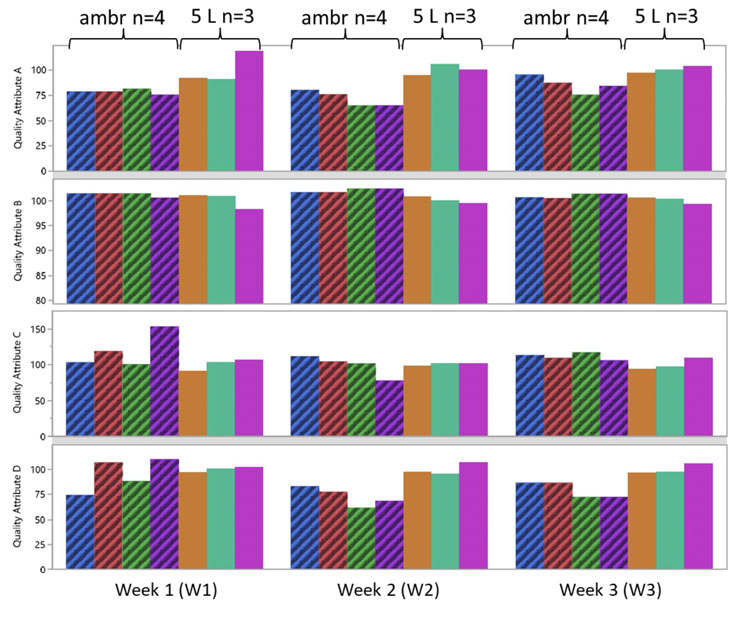
Figure 6: Quality attributes (A, B, C and D) tested for three weeks from cells expressing a coagulation factor cultured in ambr 250 HT perfusion bioreactors and 5 L benchtop bioreactors
Bioreactors for intensified processing
For seed train process intensification, SSB offers the BIOSTAT RM, a fully GMP-compliant, wave-mixed bioreactor that comes with a range of single-use bioreactor bag sizes from 2–200 L, which has a maximum 100 L liquid volume.
The RM perfusion bags are constructed of robust Flexsafe film that has proven cell growth properties. The Flexsafe RM perfusion bag features an integrated perfusion membrane at the bottom so that no external cell retention device is needed, making it very simple to operate.
To demonstrate the performance of these RM perfusion bioreactor bags, Cellca cell lines were cultured for six days in RM2, RM10, RM20 and RM50 perfusion bioreactor bags using the BIOSTAT RM platform to automate the bags and online PAT sensors, including the BioPAT ViaMass, which monitors and controls (exponentially growing) perfusion culture and allows automatic feeding and bleeding.
The results (Figure 7) showed that the RM perfusion bioreactors produced very high cell densities (≥100 x 106 cells/mL) that were scalable up to a working volume of 25 L.3,5
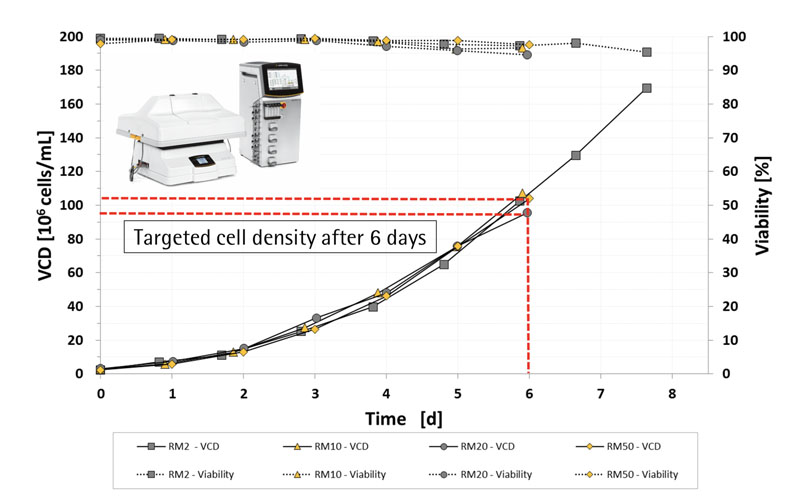
Figure 7: Viable cell density and the viability of cells cultured for 6 days in RM2, RM10, RM20 and RM50 perfusion bioreactors
Using a RM 20 perfusion bioreactor, with 100 x 106 cells/mL, operators can directly inoculate a 200 L bioreactor at a cell density of 0.5 x106 cells/mL, thereby reducing the seed train to a minimum number of stages.
However, to inoculate a production bioreactor at a higher cell density, operators can use the RM200; with 100 L of 50 x 106 cells/mL, a high cell density inoculation of 5 x 106 cells/mL in a 100 L bioreactor or 2.5 mL x 106 cells/mL in a 2000 L bioreactor can be achieved, which means this is suitable for reducing seed trains as well as implementing high cell density cell banking scenarios.
For intensified processing at bench, pilot and manufacturing scale, SSB has a range of stirred tank bioreactors, the BIOSTAT STR, which scales from 12.5 L (STR50) to 2000 L (STR2000).
These have, for several years, been capable of very high cell density culture and the BIOSTAT STR bioreactors have been shown to produce up to 200 x 106 cells/mL in concentrated fed batch conditions with CHO cell lines cultured for 12 days in 50 L and 500 L bioreactors using a tangential flow filtration (TFF) retention system.
Without any oxygen or other limitations observed, both the 50 L and 500 L stirred tank bioreactors showed comparable viable cell densities and product titres indicating scalability up to 500 L working volume.6
Downstream challenges
Intensified cell culture and high cell densities present a number of challenges in terms of primary recovery and purification operations because amassed cell numbers produce an associated increase in solids and, depending primarily on cell viability at harvest, impurities such as host cell proteins (HCPs), DNA and aggregated proteins.
This requires specific technologies that have the capacity to separate high cell densities — as well as cell debris — from the product stream and preferably some of the smaller impurities without clogging or fouling with time.
To separate the cells from the media in high cell density fed batch and concentrated fed batch cultures, SSB has the patented kSep single-use, sterile (gamma irradiated at all product contact surfaces) closed centrifuge. Unlike a classical centrifuge, it operates by balancing out centrifugal force and medium flow to keep cells contained in an expanded bed.
This only applies low shear forces to the cells to minimise lysis and HCP release. Additionally, it also dramatically reduces the filter area, does not require prerinse steps and reduces the washing buffer volumes required to efficiently separate out the cultured cells. The kSep centrifuge has been shown to efficiently clarify very high cell density mammalian cell cultures (>100 x 106 cells/mL).
For direct cell capture from perfusion permeate, SSB, in collaboration with NovaSep, offers a high capacity purification system: the BioSC platform with Multi-Column Chromatography (MCC). This flexible, modular system enables users to perform numerous unit operations on the same system in batch, parallel batch or simulated moving bed (SMB) modes.
In 2019, SSB entered a partnership with NovaSep to develop a next generation MCC system using membrane adsorbers to provide additional options to purifying product from high cell density perfusion permeates.
Conclusion
Intensified processing enables biopharmaceutical companies and CDMOs to utilise smaller single-use bioreactors and manufacturing facilities with increasingly smaller footprints. However, it does require upstream and downstream tools that are capable of culturing and processing higher cell densities.
SSB offers highly suitable solutions and services for intensified biomanufacturing from cell development through to cell separation and clarification. Cell development begins with the Cellca CHO cell lines that can be cultured to high densities in Sartorius cell culture media and a GMP cell development and cell banking service, which uses Fill-it technology to produce up to 500 x 5 mL vials containing up to 50 x106 cells/mL for process intensification of the seed train.
At the next upstream stage, process development, SSB provides its ambr 250 HT perfusion system that, for the first time, allows high throughput perfusion process development, delivering robust and optimised perfusion processes to manufacturers with substantially reduced time lines and resources.
Finally, to complete the process of upstream integration for pilot- and manufacturing-scale production, there are RM perfusion bioreactors for seed train intensification, as well as benchtop and single-use stirred tank bioreactors to achieve intensified cell cultures at up to 2000 L scale, and with proven cell densities of up to 200 x 106 cell/mL at 500 L scale.
Downstream clarification can be challenging with high cell density cultures, so SSB is developing solutions for this with its single-use kSep centrifugation and chromatography systems in partnership with NovaSep.
In summary, leveraging these integrated upstream and downstream technologies from SSB will help the biopharmaceutical industry to achieve process intensification, which could potentially accelerate manufacturing speed and productivity using smaller facilities and fewer resources to deliver better global access to more affordable biologics and vaccines.
References
3. M. Schulze, et al., “CHO Seed Culture Intensification and its Cellular Effects on N-Stage,” poster 568 presented at ESACT 2019 (www.esact2019.com/wp-content/uploads/2019/05/ESACT-2019-Poster-Abstracts.pdf).
4. B. Zoro, et al., “Development of an Automated Perfusion Bioreactor: ambr 250 Perfusion,” poster 564 presented at ESACT 2019 (www.esact2019.com/wp-content/uploads/2019/05/ESACT-2019-Poster-Abstracts.pdf).
5. J-C. Matuszczyk, et al., “Seed Train Intensification in Rocked 2D-Bioreactors Affecting CQAs,” poster 569 presented at ESACT 2019 (www.esact2019.com/wp-content/uploads/2019/05/ESACT-2019-Poster-Abstracts.pdf).
6. G. Zijlstra, “Scale-Up of Extremely High Cell Density CHO Cultures to Commercial Manufacturing Scale in Single-Use Bioreactors,” presentation at Sartorius Upstream and Downstream Forum 2014 (www.sartorius.ie/sartoriusIE/en/EUR/upstream-downstream-forum).
Part I of this article is available here.
