Talking about new science and techniques in topical formulation development, Jeremy believes that there are a number of frontiers being challenged. “When developing a topical product, understanding the overall approach is critical for reducing the risks in development,” he says: “it might be the case that a company has developed a completely new drug for an ailment or condition that needs local delivery through the skin, eye, nail, airway or mucosal membrane. Alternatively, the developers may have a drug that was previously used in an oral dosage form and has now been directed at a new local indication — so called repurposing.”
“Or, they might simply be targeting a generic application for an existing drug product. In all of these situations, our 20 years of experience in this field shows how essential it is to run performance testing (PT) alongside formulation development. Performance testing for local delivery, unlike with systemic routes, has been increasing in sophistication during this time and, in recent years, this has accelerated with the expanding knowledge of molecular and tissue biology.”
Performance testing
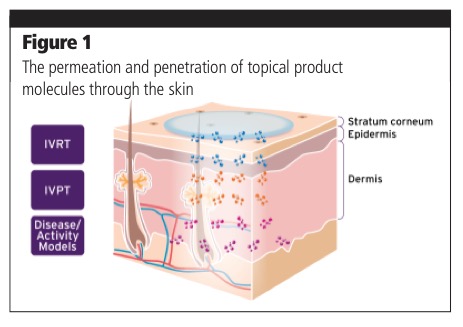
Of the three types of PT, there’s IVRT (in vitro release testing), which is relatively routine (Figure 1). “You place the topical product on a synthetic membrane in a vertical diffusion cell (VDC) and assess whether the active ingredient comes out of the formulation.”
“This is, of course, nothing new and has been around for years. What’s happening now is that this standard dissolution method for dermal drugs is not only being used to develop formulations, it’s also being applied in quality control.”
“Regulatory authorities are now demanding that companies demonstrate the ability of their formulation to pass this specification test when they manufacture at commercial scale and during stability studies. This requires the IVRT method to be validated, which is a challenge because there are lots of different variables involved.”
You have to have a certain level of experience and expertise to be able to do that. The bottom line, says Jeremy, is that many development companies are still unaware of this new and evolving trend that IVRT needs to be part of their quality control and specification procedure for new submissions. He cited a 2005 paper in which 18 laboratories tested a number of samples and there was a 35% variation in the overall result.
“So, to overcome some of the variability and operator error, MedPharm have developed and implemented an automated system (MedStat-HT). MedStat-HT is an automated system of 24 VDCs more commonly known as Franz or ‘static’ cells for measuring the in vitro release of drug from topical medicines across synthetic membranes (IVRT). Unique design features along with automated collection provide a significant reduction in the variation associated with sampling and fluidics compared with manually operated VDC systems.
The classic IVRT procedure is that the technician takes a sample, runs it through HPLC, replaces the reservoir with a fluid and then, in an hour or two, takes another sample. Now we just auto-sample into 96-well plates; we can measure the diffusion at many more time points and, as a result, get much tighter confidence limits. The key benefits of the new system are its ability to better characterise formulation drug release properties for potential patent claim support, formulation optimisation and comparison, and the development of product quality and stability specifications with a reduction in experimental time. It just goes to show, says Jeremy, that even the old, established methods are being pushed forward and taken to the next level to de-risk development programmes, reduce costs and enhance quality.
A more sophisticated assessment of the performance of a topical product is IVPT (in vitro permeation testing). In this instance, instead of using a synthetic membrane, you actually use human skin. You place the topical on the skin, which is held in the VDC, and monitor how much of the drug penetrates and permeates the skin, and how much remains unabsorbed. Although synthetic skin is available (as is rodent or pig skin), MedPharm always uses human donor skin from cosmetic surgeries such as tummy tucks as a more accurate experimental model. The company also has tests for eyes, but whereas human skin is easy to acquire, human eyes are a little less accessible. As such, pig eyes are used as a substitute until later-stage testing is required.
A number of different factors can affect skin permeability, such as the number of hair follicles, origin of the skin on the body and ethnicity. For example, darker skin tones are less hydrated and, therefore, topical formulations permeate differently compared with lighter skin. MedPharm generally uses female skin because it has fewer follicles, but occasionally uses male skin when high follicular density is required. Therefore, although donor to donor variation remains a challenge, experience has taught the company that this can be minimised with standard operating procedures and careful handling.
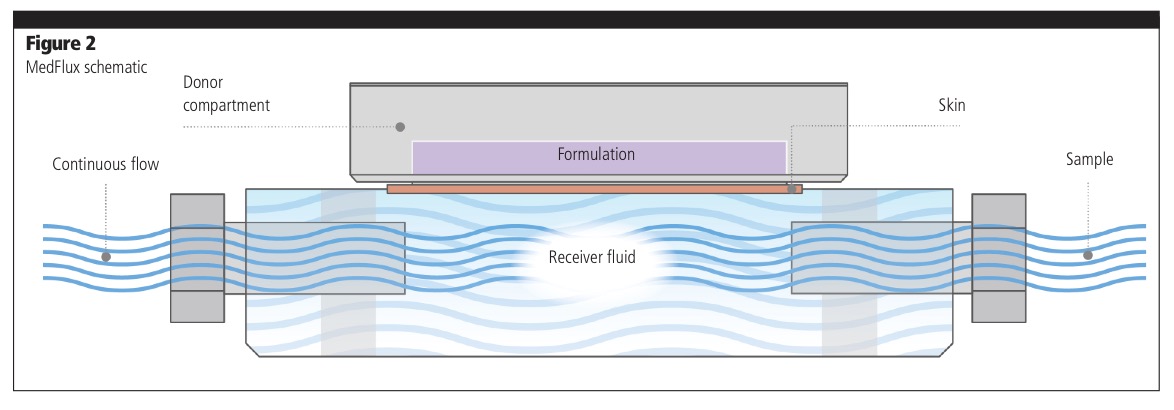
“At the human skin testing stage, you’re moving away from HPLC analysis because the concentration levels are much lower and you need to employ bioanalytical techniques such as LC-MS to measure the amount of drug reaching the different layers of the skin and also through the skin into the receiver fluid. Again, MedPharm has been pushing the boundaries of the technology and, instead of having a static reservoir under the skin in the VDC, we have created a flow-through system underneath it so, particularly with lipophilic drugs, you don’t get to a point when the reservoir is saturated. It’s much more closely aligned to what happens in a clinical setting.”
The technology we’ve developed is called MedFlux-HT (Figure 2), which is an automated system of 24 flow-through diffusion cells to measure IVPT. The system has been uniquely designed to provide local tissue clearance to mimic the clinical setting and maximise sink conditions without the addition of solvents required when studying lipophilic compounds in static cells. It is an automated high throughput system wherein samples are taken directly into 96 well plates and then straight to the LC-MS. This dramatically reduces variability compared with static, manual methods.
“More recently MedPharm’s skin biology experts have developed methods that enable us to keep fresh skin, which we use within 24 hours of it being removed from the donor, healthy in the laboratory environment for about 10 days. We create disease activity models by inducing inflammation pathways that are associated with key skin diseases, such as psoriasis and atopic dermatitis, and monitoring the associated biomarkers. We can watch the formulation penetrate the skin (pharmacokinetics) and, by monitoring the biomarkers, measure its activity (pharmacodynamics). So, you get an activity readout as well as an indication of where the effect is happening. This is powerful information when making the multi-million dollar decision to invest in a clinical trial.” As new pathways are identified, we’re developing new models all the time, notes Jeremy. “Thus, to further de-risk the assessment we can follow the impact of a drug on multiple pathways depending on what indication is being targeted.”
Client benefits
“How this is benefiting our customers is, for example, when they’re trying to repurpose a new drug into a skin application, they may not have patent coverage on the molecule itself. Although a patent might exist for the route of delivery, we can help to prove that one formulation is better than another. We’ve seen some surprising effects between the excipients and the active that have improved skin permeation and penetration.”
“We’re using these tests to both enhance the formulation development process and de-risk the “proceed to clinical trial” decision by giving the client data that they can present to their investors or senior management — and the regulatory authorities — to support the safety and efficacy of their product. For example, we’ve been able to redevelop formulations from 1% to 0.1% active based on different release rates and the incorporation of different ingredients.”
Regulatory authorities are also starting to accept demonstrations of bioequivalence using these methods in lieu of expensive clinical trials in generic applications. This is opening up many markets for these products to generic competition because it brings the return of investment into acceptable limits.
“Using these methods, we’re providing a scientifically robust way to prove that a new, repurposed or generic product can be used effectively through topical routes of delivery.”
Taking all these developments together, Jeremy concludes that topical formulation has a bright future: “Demand for topical products is booming while, at the same time, our knowledge of skin chemistry and ability to develop, test and manufacture new products to the highest standards is always expanding. Add in the growing trend to repurpose existing oral drugs for topical delivery and you can see why this is an exciting period for MedPharm.”
