The non-adherence of inhaled therapies in asthma and chronic obstructive pulmonary disease (COPD) is known to be associated with poor disease control, reduced quality of life, increased exacerbations and higher healthcare utilisation.
Adherence to inhaled therapy is estimated to be 70–90% in randomised, controlled trials but it can be as low as 10–40% in a clinical setting.7
Electronic sensors can be added to capsule-based inhalers to mitigate this problem. The electronic Breezhaler, for example, is a capsule-based, single unit dose dry powder inhaler with integrated electronics to monitor device usage. Handling and drug delivery performance have been shown to be equivalent to that of the marketed Breezhaler without the sensors.
The idea behind this development is the difference between the audio signal of a successful or unsuccessful inhalation (Figure 5). The device has a microphone that senses the rattling noise made by the spinning capsule when the patient inhales through the device.
Once the rattle is detected, an algorithm processes the raw acoustic data to generate meaningful inhalation usage data (inhalation performance profile and summary of inhalation event) and a relative timestamp, which are stored on the device. Such data can provide the patient feedback required to drive improved inhaler technique. These methods may be useful for training purposes or trending when a patient receives a new device.7
Easy drug development process
The first point to consider is the device cost. The inhaler cost depends mainly on the number of high precision parts needed and assembly. Reservoir and blister-based inhalers require more than 20 plastic and metal parts, whereas capsule-based inhalers are composed of 6–10 parts (including the pins).
In comparison, capsule-based inhalers are cheaper and simple to use (Figure 6).
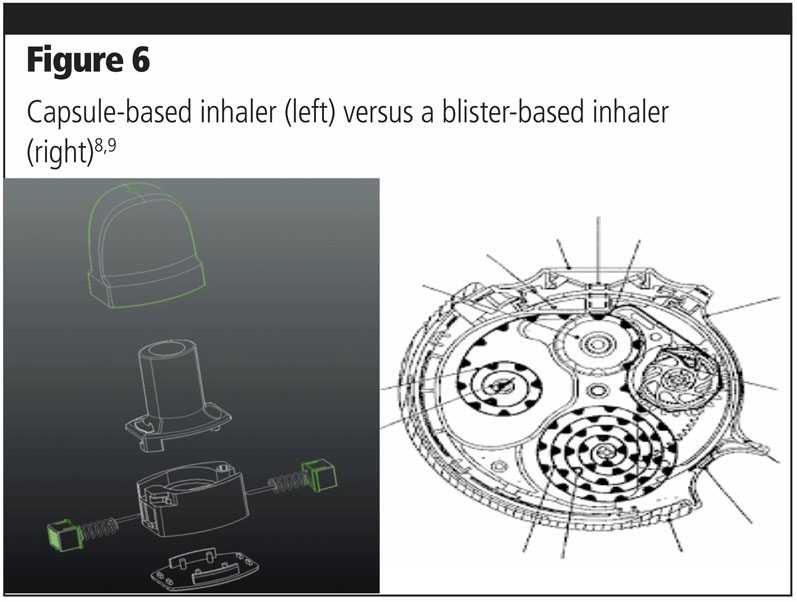
Another point is the filling of the drugs into devices compared with capsule filling. All encapsulators are standardised and reliable. It would take a relatively low investment from pharma companies to add this capability to existing equipment or purchase new models. Additionally, this allows an easier transition from the clinical, preindustrial phase to commercial.
Capsule-based inhalers may be considered to be a simple and cost-effective solution for generic medicines. Lowering variables and the cost of innovative product formulation for small niche items with a reliable option is key. Filling the capsules in a reproducible way gives drug developers a ready-to-use application instantly.
Researchers are free to concentrate their efforts and investments on the formulation themselves, as the capsules can be easily placed into a DPI.8
Environmentally friendly
Another significant advantage of capsules-based inhalers (all DPIs) is their contribution to reduced greenhouse gas (GHG) emissions. It has been a decade since the 2008 passage of the Climate Change Act in the United Kingdom.
Despite this, atmospheric carbon dioxide levels have increased, surpassing 400 parts per million. Although the potential impact of climate change on respiratory health is widely appreciated, it is unknown to many that respiratory drugs may be contributing to global warming. This is because of propellant gases used in some metered dose inhalers (MDIs). DPIs for the delivery of respiratory drugs can reduce the source of greenhouse gas emissions.10
A product carbon footprint (PCF) summarises and characterises the climate change impact of a product by assessing GHG emissions during the product lifecycle. The PCF for Spiriva is 0.78 kg CO2 equivalents whereas Atrovent and Berodual (metered dose inhalers) have 14.59 and 16.48 kg CO2 equivalents, respectively.11
In conclusion
There are many platforms available for inhalation technology. They are classified into three categories: nebulisers, metered dose inhalers and dry powder inhalers. We cannot advise that any one in particular is the best, as it depends on the system and the drug formulation chosen. The characteristics of the specific system will define which one is more convenient to use.
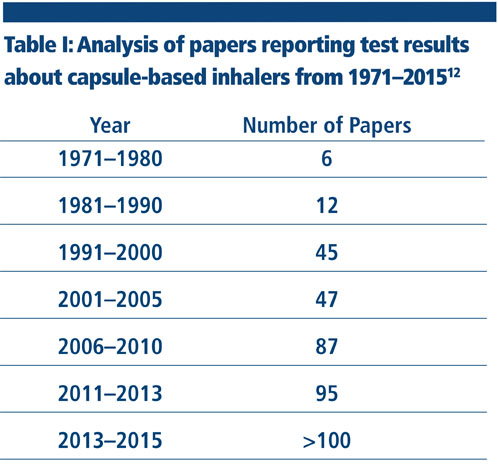
Capsule-based inhalers belong to the category of DPIs. They are a convenient option for formulators as described in this paper. In these scenarios, capsule-based inhalers offer significant advantages compared with other platforms. This explains why there is a growing level of interest in this delivery method throughout the pharmaceutical community (Table I).
References
- 7. P. Colthorpe, et al., “Adding Electronics to the Breezhaler: Satisfying the Needs of Patients and Regulators,” Respiratory Drug Delivery 1, 71–80 (2018).
- 8. P. Magni, “Single-Dose Inhalers for Capsules: A Consolidated Tradition with Ample Growth Prospects,” www.ondrugdelivery.com/publications/Pulmonary%20Nasal%20November%202012/Plastiape.pdf.
- 9. H. Christin, “The Diskus: A Review of its Position Among Dry Powder Inhaler Devices,” International Journal of Clinical Practice 61, 1022–1036 (2007).
- 10. B.E. Jones and F. Diez, “Advantages of Capsule-Based Dry Powder Inhalers,” Inhalation (December 2015) pp 1–5.
- 11. M. Hansel, et al., “A Comparison of Product Carbon Footprints of Respimat versus Pressurized Metered Dose Inhalers,” Drug Delivery 2, 321–324 (2018).
- 12. ACG, “Capsule-Based Dry Powder Inhalation: A Holistic Approach for Effective Pulmonary Drug Delivery,” https://business-review-webinars.com/webinar/Pharma/Capsule_Based_Dry_Powder_Inhalation_A_Holistic_Approach_for_Effective_Pulmonary_Drug_Delivery-G7BmrbCM.
Click here to read part I.
