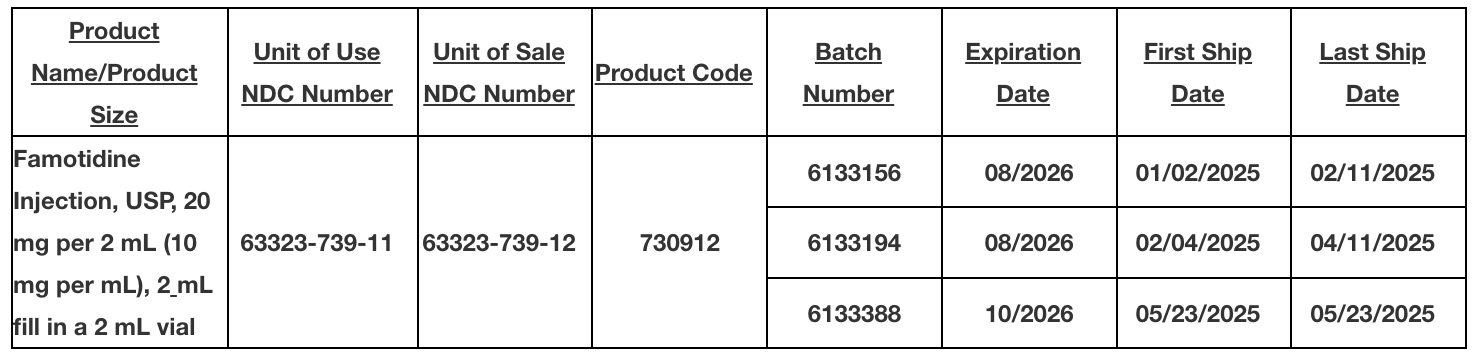
Fresenius Kabi is voluntarily recalling three lots (numbers 6133156, 6133194, 6133388) of Famotidine Injection, USP, 20 mg per 2 mL (10 mg per mL), 2 mL fill in a 2 mL vial.
This recall is being performed at the user level in the United States.
The product is being recalled due to out-of-specification (OOS) endotoxin results of certain reserve samples from a single lot.
Based upon the investigation, two additional lots were also included in the recall as a precautionary measure.
Elevated endotoxin levels can precipitate severe systemic reactions such as sepsis and septic shock.
Severe responses may include inflammatory and life-threatening immune responses and death.
Non-serious adverse event reports potentially associated with the OOS have been received for one lot.
These non-serious adverse events included chills, change in mental status, change in respiratory status, fever, increase in body temperature, shivering and shaking.
Famotidine Injection is indicated in some hospitalised patients with pathological hypersecretory conditions or intractable ulcers, or as an alternative to the oral dosage forms for short-term use in patients who are unable to take oral medication for the following conditions:
- Short-term treatment of an active duodenal ulcer
- Maintenance therapy for duodenal ulcer patients at reduced dosage after healing of an active ulcer
- Short-term treatment of an active benign gastric ulcer
- Short-term treatment of gastroesophageal reflux disease (GERD)
- Treatment of pathological hypersecretory conditions.
- Fresenius Kabi is notifying its distributors and customers and is arranging for the return of the recalled product.
If health care facilities have any of the affected lots, they are to immediately discontinue distributing, dispensing or using the lots and return all units to Fresenius Kabi.
Distributors are instructed to immediately notify their customers who have been shipped or may have been shipped the product involved in this recall.
This recall is being conducted with the knowledge of the US Food and Drug Administration.
